Melatonin prevents cyclophosphamide-induced primordial follicle loss by inhibiting ovarian granulosa cell apoptosis and maintaining AMH expression
- PMID: 35992124
- PMCID: PMC9381702
- DOI: 10.3389/fendo.2022.895095
Melatonin prevents cyclophosphamide-induced primordial follicle loss by inhibiting ovarian granulosa cell apoptosis and maintaining AMH expression
Abstract
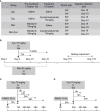
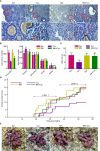
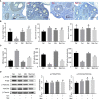
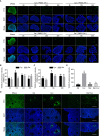
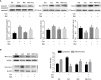
Cyclophosphaty -45mide (Cyc) chemotherapy in young female cancer patients is associated with an increased risk of premature ovarian insufficiency (POI). This study was designed to investigate the protective role of melatonin (Mel) as an adjuvant against Cyc-induced POI. Female mice received a single intraperitoneal (i.p.) dose of Cyc (75 mg/kg). Mel protection was achieved in mice after i.p. injection of melatonin (50 mg/kg) every 24 h for four consecutive days prior to chemotherapy initiation and for 14 additional days. Ovarian reserve testing, hormonal assays for follicle-stimulating hormone, luteinizing hormone, and anti-Müllerian hormone (AMH), assessment of the oxidative stress status, and measurement of the relative expression of genes in PTEN/AKT/FOXO3a and mitochondrial apoptosis pathways were performed. The results showed that treatment with 50 mg/kg Mel significantly prevented Cyc-induced over-activation of primordial follicles by maintaining the plasma level of AMH and subsequently preventing litter size reduction in mice treated with Cyc chemotherapy. Importantly, Mel treatment significantly prevented ovarian granulosa cell loss by inhibiting the mitochondrial apoptotic pathway. Identifying the protective actions of Mel against Cyc-induced primordial follicle loss has important implications for fertility maintenance in young cancer patients undergoing chemotherapy.
Keywords: anti-Mullerian hormone; apoptosis; cyclophosphamide; granulosa cell; melatonin; primordial follicle.
Copyright © 2022 Feng, Ma, Li, Pei, Deng, Jia and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

