Isolation, characterization, and functional study of extracellular vesicles derived from Leishmania tarentolae
- PMID: 35992172
- PMCID: PMC9381964
- DOI: 10.3389/fcimb.2022.921410
Isolation, characterization, and functional study of extracellular vesicles derived from Leishmania tarentolae
Abstract
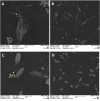
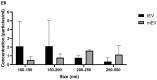
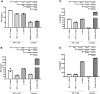
Leishmania (L.) species are protozoan parasites with a complex life cycle consisting of a number of developmental forms that alternate between the sand fly vector and their host. The non-pathogenic species L. tarentolae is not able to induce an active infection in a human host. It has been observed that, in pathogenic species, extracellular vesicles (EVs) could exacerbate the infection. However, so far, there is no report on the identification, isolation, and characterization of L. tarentolae EVs. In this study, we have isolated and characterized EVs from L. tarentolae GFP+ (tEVs) along with L. major GFP+ as a reference and positive control. The EVs secreted by these two species demonstrated similar particle size distribution (approximately 200 nm) in scanning electron microscopy and nanoparticle tracking analysis. Moreover, the said EVs showed similar protein content, and GFP and GP63 proteins were detected in both using dot blot analysis. Furthermore, we could detect Leishmania-derived GP63 protein in THP-1 cells treated with tEVs. Interestingly, we observed a significant increase in the production of IFN-γ, TNF-α, and IL-1β, while there were no significant differences in IL-6 levels in THP-1 cells treated with tEVs following an infection with L. major compared with another group of macrophages that were treated with L. major EVs prior to the infection. Another exciting observation of this study was a significant decrease in parasite load in tEV-treated Leishmania-infected macrophages. In addition, in comparison with another group of Leishmania-infected macrophages which was not exposed to any EVs, tEV managed to increase IFN-γ and decrease IL-6 and the parasite burden. In conclusion, we report for the first time that L. tarentolae can release EVs and provide evidence that tEVs are able to control the infection in human macrophages, making them a great potential platform for drug delivery, at least for parasitic infections.
Keywords: Leishmania major; Leishmania tarentolae; extracellular vesicles; human macrophage cell line; infection.
Copyright © 2022 Shokouhy, Sarvnaz, Taslimi, Lajevardi, Habibzadeh, Mizbani, Shekari, Behbahani, Torrecilhas and Rafati.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Al-Kamel M. A. (2017). Stigmata in cutaneous leishmaniasis: Historical and new evidence-based concepts. Our Dermatol. Online/Nasza Dermatol Online 8 (1), 81–90. doi: 10.7241/ourd.20171.21 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

