Expression of PD-L1 as a predictive marker of sensitivity to immune checkpoint inhibitors in patients with advanced biliary tract cancer
- PMID: 35992188
- PMCID: PMC9386848
- DOI: 10.1177/17562848221117638
Expression of PD-L1 as a predictive marker of sensitivity to immune checkpoint inhibitors in patients with advanced biliary tract cancer
Abstract
Background: Expression of programmed death-ligand 1 (PD-L1) has been reported to correlate with response to immune checkpoint inhibitors (ICIs) in various tumor types. However, there are few data on the role of PD-L1 expression as a predictive and prognostic biomarker of sensitivity to ICIs in patients with advanced biliary tract cancer (BTC).
Objectives: We evaluated the role of PD-L1 expression as a predictive and prognostic biomarker of response to ICIs in patients with advanced BTC.
Design: We retrospectively analyzed data from 83 advanced BTC patients who received ICIs as second- or third-line treatment between February 2018 and April 2021.
Methods: All patient data analysis included evaluation of PD-L1 expression by the combined positive score (CPS).
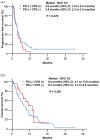
Results: Among 83 patients, 56 (67.5%) had PD-L1 positivity (CPS ⩾ 1). The objective response rate (ORR) to ICIs was significantly higher in advanced BTC patients with PD-L1 expression compared to those without PD-L1 expression (17.8% versus 0%, p = 0.026). However, there were no significant differences in median progression-free survival (PFS; 2.9 versus 2.6 months, p = 0.330) and median overall survival (OS; 8.1 versus 6.3 months, p = 0.289) as a response to ICIs between patients with and without PD-L1 expression. Also, there were no significant differences in ORR, PFS, and OS as a response to ICIs in conjunction with a response to a prior gemcitabine plus cisplatin regimen (p = 0.654, p = 0.278, and p = 0.302, respectively).
Conclusions: The present study suggests that the expression of PD-L1 alone was not sufficient as a novel marker to select advanced BTC patients who might benefit from ICIs. Additional comprehensive studies of biomarkers that can assist in predicting BTC patient responses to pembrolizumab and/or nivolumab therapy are required.
Keywords: biliary tract neoplasms; biomarker; chemotherapy; immunotherapy; programmed death-ligand 1 expression.
© The Author(s), 2022.
Conflict of interest statement
Competing interests: The authors declare that there is no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

