Sustainable spectrophotometric determination of antihypertensive medicines reducing COVID-19 risk via paired wavelength data processing technique - Assessment of purity, greenness and whiteness
- PMID: 35992213
- PMCID: PMC9376343
- DOI: 10.1016/j.scp.2022.100806
Sustainable spectrophotometric determination of antihypertensive medicines reducing COVID-19 risk via paired wavelength data processing technique - Assessment of purity, greenness and whiteness
Abstract
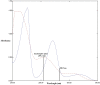
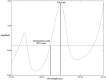
Recent studies have reported that using certain antihypertensive therapies such as angiotensin II receptor blockers (ARBs) is associated with mitigation of fatal outcomes and enhancing clinical features of patients having hypertension during coronavirus pandemic. Thus, in the current work an innovative, effective, white and sustainable spectrophotometric technique called paired wavelength data processing technique (PWDPT) was developed for evaluation of recommended antihypertensive combination therapies incorporating candesartan cilexetil (CAN) and hydrochlorothiazide (HCT). This technique included three methods, namely, absorbance resolution (AR), amplitude resolution (PR) and ratio extraction (RE). Linearity ranges were (5.0 μg/mL - 50.0 μg/mL) and (2.0 μg/mL - 24.0 μg/mL) for CAN and HCT, respectively. Validation and confirmation of all suggested methods were conducted in accordance with ICH guidelines, producing satisfactory results within the accepted limits. Statistical comparison was achieved between the attained results from suggested methods and those attained from official methods, in which insignificant difference was existed. The suggested methods were successfully employed for identification of the studied drugs as well as determination of their spectral recognition and evaluation of the purity in their combined formulations. The proposed methods followed the principles of green analytical chemistry, where their greenness was evaluated and compared with the official potentiometric and HPLC methods via using four tools, namely, National Environmental Methods Index (NEMI), the Analytical Eco-Scale, the Green Analytical Procedure Index (GAPI) and Analytical greenness metric (AGREE) which affirmed the eco-friendly nature of the proposed methods. Moreover, studying the whiteness features was performed using the recently introduced RGB12 model. The acceptable results along with the sustainability, simplicity, affordability and low-cost of the proposed methods encourages their utilization in the quality control laboratories.
Keywords: Absorbance resolution (AR); Amplitude resolution (PR) and ratio extraction (RE); Candesartan cilexetil (CAN); Hydrochlorothiazide (HCT).
© 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

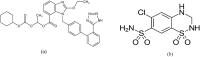

References
-
- Abdelaleem E., Naguib I., Zaazaa H., Draz M. Simultaneous determination of some antihypertension drugs in their binary mixtures by simple spectrophotometric methods. Asian J Biomed. Pharm. Sci. 2013;3:5–12.
-
- Ahmad Charoo N., Bashir M., Abdalla E., Ali K.I.H. Determination of candesartan cilexetil in tablet dosage forms and dissolution testing samples by first derivative UV spectrophotometric method. Anal. Lett. 2009;42:2232–2243.
-
- Ahmed D.A., Lotfy H.M. Evaluation of in silico and in lab sample enrichment techniques for the assessment of challengeable quaternary combination in critical ratio. Spectrochim. Acta Mol. Biomol. Spectrosc. 2021;260 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
