Disparities in hepatitis C care across Canadian provincial prisons: Implications for hepatitis C micro-elimination
- PMID: 35992251
- PMCID: PMC9202774
- DOI: 10.3138/canlivj-2020-0035
Disparities in hepatitis C care across Canadian provincial prisons: Implications for hepatitis C micro-elimination
Abstract
Background: Delivery of hepatitis C virus (HCV) care to people in prison is essential to HCV elimination. We aimed to describe current HCV care practices across Canada's adult provincial prisons.
Methods: One representative per provincial prison health care team (except Ontario) was invited to participate in a web-based survey from January to June 2020. The outcomes of interest were HCV screening and treatment, treatment restrictions, and harm reduction services. The government ministry responsible for health care was determined. Non-nominal data were aggregated by province and ministry; descriptive statistical analyses were used to report outcomes.
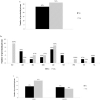
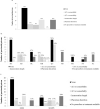
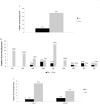
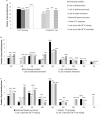
Results: The survey was completed by 59/65 (91%) prisons. On-demand, risk-based, opt-in, and opt-out screening are offered by 19 (32%), 10 (17%), 18 (31%), and 9 (15%) prisons, respectively; 3 prisons offer no HCV screening. Liver fibrosis assessments are rare (8 prisons access transient elastography, and 15 use aspartate aminotransferase to platelet ratio or Fibrosis-4); 20 (34%) prisons lack linkage to care programs. Only 32 (54%) prisons have ever initiated HCV treatment on site. Incarceration length and a fibrosis staging of ≥F2 are the most common eligibility restrictions for treatment. Opioid agonist therapy is available in 83% of prisons; needle and syringe programs are not available anywhere. Systematic screening and greater access to treatment and harm reduction services are more common where the Ministry of Health is responsible.
Conclusions: Tremendous variability exists in HCV screening and care practices across Canada's provincial prisons. To advance HCV care, adopting opt-out screening and removing eligibility restrictions may be important initial strategies.
Keywords: elimination; hepatitis C virus (HCV); linkage to care; prison; screening; treatment.
Copyright © 2021 Canadian Association for the Study of the Liver.
Conflict of interest statement
CD, BM, and CWN have no reported conflicts. NK has received research funding from Gilead Sciences, advisory fees from Gilead Sciences, ViiV Healthcare, Merck and Abbvie, and speaker fees from Gilead Sciences and Merck. SB is an (unpaid) non–executive director of Canadian registered charity Hepatitis Education and Prevention Society of British Columbia, an (unpaid) executive director of Papua New Guinean and Australian registered charity Grass Skirt Project Inc, and has received advisory fees from Gilead Sciences and speaker fees from Gilead Sciences (all personal fees given as unrestricted donations to BCCDC Foundation for Public Health). DF has received advisory fees from Gilead Sciences, Merck, and Abbvie. KK has received advisory fees from Gilead Sciences and Abbvie. KH has received research funding and speaker fees from Gilead Sciences and is employed by a non-profit funded in part by Gilead Sciences, Abbvie, and Merck. JC has received consulting fees from ViiV Healthcare and Gilead, grants from ViiV Healthcare, Merck, and Gilead; and payment for lectures from Gilead.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous
