Bacterial hemerythrin domain-containing oxygen and redox sensors: Versatile roles for oxygen and redox signaling
- PMID: 35992274
- PMCID: PMC9388753
- DOI: 10.3389/fmolb.2022.967059
Bacterial hemerythrin domain-containing oxygen and redox sensors: Versatile roles for oxygen and redox signaling
Abstract
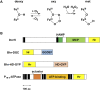
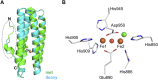
Hemerythrin is an oxygen-binding protein originally found in certain marine invertebrates. Oxygen reversibly binds at its non-heme diiron center, which consists of two oxo-bridged iron atoms bound to a characteristic conserved set of five His residues, one Glu residue, and one Asp residue. It was recently discovered that several bacteria utilize hemerythrin as an oxygen- and redox-sensing domain in responding to changes in cellular oxygen concentration or redox status, and immediately adapt to these environmental changes in order to maintain important physiological processes, including chemotaxis and c-di-GMP synthesis and degradation. This Mini Review focuses on the recent progress made on structural and functional aspects of these emerging bacterial hemerythrin domain-containing oxygen and redox sensors, revealing characteristic features of this family of proteins.
Keywords: c-di-GMP; hemerythrin; methyl-accepting chemotaxis protein; non-heme diiron; oxygen senor; redox sensor; signaling.
Copyright © 2022 Kitanishi.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Identification and Characterization of a Redox Sensor Phosphodiesterase from Ferrovum sp. PN-J185 Containing Bacterial Hemerythrin and HD-GYP Domains.Biochemistry. 2020 Mar 3;59(8):983-991. doi: 10.1021/acs.biochem.0c00021. Epub 2020 Feb 20. Biochemistry. 2020. PMID: 32045213
-
Diversity and distribution of hemerythrin-like proteins in prokaryotes.FEMS Microbiol Lett. 2008 Feb;279(2):131-45. doi: 10.1111/j.1574-6968.2007.01011.x. Epub 2007 Dec 12. FEMS Microbiol Lett. 2008. PMID: 18081840 Review.
-
Crystal structure, exogenous ligand binding, and redox properties of an engineered diiron active site in a bacterial hemerythrin.Inorg Chem. 2013 Nov 18;52(22):13014-20. doi: 10.1021/ic401632x. Epub 2013 Nov 4. Inorg Chem. 2013. PMID: 24187962 Free PMC article.
-
Diversity of structures and functions of oxo-bridged non-heme diiron proteins.Arch Biochem Biophys. 2021 Jul 15;705:108917. doi: 10.1016/j.abb.2021.108917. Epub 2021 May 12. Arch Biochem Biophys. 2021. PMID: 33991497 Free PMC article. Review.
-
A bacterial hemerythrin domain regulates the activity of a Vibrio cholerae diguanylate cyclase.Biochemistry. 2012 Oct 30;51(43):8563-70. doi: 10.1021/bi3011797. Epub 2012 Oct 18. Biochemistry. 2012. PMID: 23057727 Free PMC article.
Cited by
-
Circular intermediate-mediated horizontal transfer of the chromosome-encoded cfr(C) gene in multi-drug resistant Campylobacter coli from swine sources.Front Microbiol. 2023 Dec 22;14:1274245. doi: 10.3389/fmicb.2023.1274245. eCollection 2023. Front Microbiol. 2023. PMID: 38188581 Free PMC article.
-
Gas and light: triggers of c-di-GMP-mediated regulation.FEMS Microbiol Rev. 2023 Jul 5;47(4):fuad034. doi: 10.1093/femsre/fuad034. FEMS Microbiol Rev. 2023. PMID: 37339911 Free PMC article. Review.
-
The Oxidative Stress-Induced Hypothetical Protein PG_0686 in Porphyromonas gingivalis W83 Is a Novel Diguanylate Cyclase.Microbiol Spectr. 2023 Jan 31;11(2):e0441122. doi: 10.1128/spectrum.04411-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36719196 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

