Rapid and Economical Drug-Eluting IOL Preparation via Thermoresponsive Agarose Coating for Effective Posterior Capsular Opacification Prevention
- PMID: 35992334
- PMCID: PMC9388942
- DOI: 10.3389/fbioe.2022.930540
Rapid and Economical Drug-Eluting IOL Preparation via Thermoresponsive Agarose Coating for Effective Posterior Capsular Opacification Prevention
Abstract
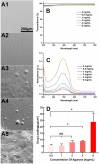
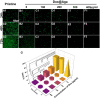
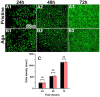
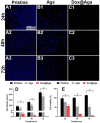
Posterior capsular opacification (PCO), the highest incidence complication after cataract surgery, is mainly due to the attachment, proliferation, and migration of the residual lens epithelial cells (LECs). Although the drug-eluting IOLs have been proved to be an effective way to prevent PCO incidence, its preparations are time consuming and require tedious preparation steps. Herein, the thermoreversible agarose is adopted to prepare drug-eluting IOL. Such functional coating can be obtained easily by simple immersion in the antiproliferative drug containing hot agarose and taken out for cooling, which not only does not affect the optical property but also can effectively decrease the PCO incidence after intraocular implantation. As a result, the proposed agarose coating provides a rapid and economical alternative of drug-eluting IOL fabrication for PCO prevention.
Keywords: agarose; drug-eluting coating; intraocular lens; posterior capsular opacification; surface modification.
Copyright © 2022 Chen, Qin, Fang, Duo, Wang, Deng, Chen and Lin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









References
-
- García-Martínez L., Campos F., Godoy-Guzmán C., del Carmen Sánchez-Quevedo M., Garzón I., Alaminos M., et al. (2017). Encapsulation of Human Elastic Cartilage-Derived Chondrocytes in Nanostructured Fibrin-Agarose Hydrogels. Histochem Cell Biol. 147 (1), 83–95. 10.1007/s00418-016-1485-9 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

