Postthrombotic syndrome and quality of life after deep vein thrombosis in patients treated with edoxaban versus warfarin
- PMID: 35992565
- PMCID: PMC9248314
- DOI: 10.1002/rth2.12748
Postthrombotic syndrome and quality of life after deep vein thrombosis in patients treated with edoxaban versus warfarin
Abstract
Background: Postthrombotic syndrome (PTS) is a long-term complication after deep vein thrombosis (DVT) and can affect quality of life (QoL). Pathogenesis is not fully understood but inadequate anticoagulant therapy with vitamin K antagonists is a known risk factor for the development of PTS.
Objectives: To compare the prevalence of PTS after acute DVT and the long-term QoL following DVT between patients treated with edoxaban or warfarin.
Methods: We performed a long-term follow-up study in a subset of patients with DVT who participated in the Hokusai-VTE trial between 2010 and 2012 (NCT00986154). Primary outcome was the prevalence of PTS, defined by the Villalta score. The secondary outcome was QoL, assessed by validated disease-specific (VEINES-QOL) and generic health-related (SF-36) questionnaires.
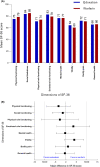
Results: Between 2017 and 2020, 316 patients were enrolled in 26 centers in eight countries, of which 168 (53%) patients had been assigned to edoxaban and 148 (47%) to warfarin during the Hokusai-VTE trial. Clinical, demographic, and thrombus-specific characteristics were comparable for both groups. Mean (SD) time since randomization in the Hokusai-VTE trial was 7.0 (1.0) years. PTS was diagnosed in 85 (51%) patients treated with edoxaban and 62 (42%) patients treated with warfarin (adjusted odds ratio 1.6, 95% CI 1.0-2.6). Mean differences in QoL scores between treatment groups were not clinically relevant.
Conclusion: Contrary to our hypothesis, the prevalence of PTS tended to be higher in patients treated with edoxaban compared with warfarin. No differences in QoL were observed. Further research is warranted to unravel the role of anticoagulant therapy on development of PTS.
Keywords: edoxaban; postthrombotic syndrome; quality of life; venous thrombosis; warfarin.
© 2022 The Authors. Research and Practice in Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis (ISTH).
Conflict of interest statement
J.B.W. reports personal fees and other from Bayer HealthCare, personal fees and other from Boehringer. Ingelheim, personal fees and other from BMS/Pfizer, personal fees and other from CSL Behring, personal fees and other personal fees and other from Daiichi Sankyo, personal fees and other from LEO Pharma, outside the submitted work. F.C. reports grants from BMS/Pfizer, personal fees and other from Bayer HealthCare, personal fees and other from AstraZeneka, personal fees and other from MSD, personal fees and other from GSK, other from Janssen, personal fees and other from Novartis, outside the submitted work. M.C. reports personal fees from Bayer HealthCare, personal fees from Boehringer Ingelheim, personal fees from Bristol‐Myers Squib, personal fees from CSL Behring, personal fees from Daiichi Sankyo, personal fees from Pfizer, personal fees from Portola, personal fees from Sanquin Blood Supply, outside the submitted work. W.G. reports grants and other from Bayer HealthCare, grants and other from Pfizer, other from Novartis, other from Amgen, other from Principia, from Sanofi, other from MSD, other from Sobi, outside the submitted work. K. Meijer receives other from Bayer HealthCare, other from Uniqure, other from Alexion, other from Octapharma, outside the submitted work. S.M. reports grants from GSK, grants from BMS/Pfizer, grants from Aspen, grants from Daiichi Sankyo, grants from Bayer HealthCare, grants from Boehringer Ingelheim, grants from Sanofi, grants from Portola, outside the submitted work. M.A.S.P. reports honoraria from Bayer, Pfizer, and Leo Pharma. O.S. reports grants from Daiichi‐Sankyo, during the conduct of the study; grants, personal fees, and nonfinancial support from Bayer HealthCare, grants, personal fees and nonfinancial support from BMS, personal fees and non‐financial support from Pfizer, grants, personal fees and non‐financial support from Boehringer Ingelheim, grants and personal fees from MSD, personal fees from Chiesi, grants and personal fees from Boston Scientifics, outside the submitted work. S.M.S. reports receiving consulting fees from Bayer and Boehringer Ingelheim, and lecture fees from Bayer, Boehringer Ingelheim, and Bristol‐Myers Squibb–Pfizer. P.V. reports grants and personal fees from Bayer HealthCare, grants and personal fees from Boehringer Ingelheim, grants and personal fees from BMS/Pfizer, personal fees from Daiichi Sankyo, personal fees from LEO Pharma, personal fees from Anthos therapeutics, personal fees from Portola Pharmaceuticals/Alexion, outside the submitted work. M.G., M.S., and Y.L. report being an employee of Daiichi‐Sankyo. No other potential conflict of interest with relation to this study were reported.
Figures
References
-
- Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal‐vein thrombosis. Lancet. 1997;349(9054):759‐762. - PubMed
-
- Prandoni P, Lensing AW, Cogo A, et al. The long‐term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1‐7. - PubMed
-
- Rabinovich A, Kahn SR. How I treat the postthrombotic syndrome. Blood. 2018;131(20):2215‐2222. - PubMed
-
- Kahn SR. How I treat postthrombotic syndrome. Blood. 2009;114(21):4624‐4631. - PubMed
-
- Prandoni P, Lensing AW, Prins MH, et al. Below‐knee elastic compression stockings to prevent the post‐thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141(4):249‐256. - PubMed
LinkOut - more resources
Full Text Sources
Medical

