DDX5 and DDX17-multifaceted proteins in the regulation of tumorigenesis and tumor progression
- PMID: 35992805
- PMCID: PMC9382309
- DOI: 10.3389/fonc.2022.943032
DDX5 and DDX17-multifaceted proteins in the regulation of tumorigenesis and tumor progression
Erratum in
-
Corrigendum: DDX5 and DDX17-multifaceted proteins in the regulation of tumorigenesis and tumor progression.Front Oncol. 2023 Jun 2;13:1204712. doi: 10.3389/fonc.2023.1204712. eCollection 2023. Front Oncol. 2023. PMID: 37333828 Free PMC article.
Abstract
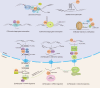
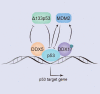
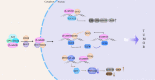
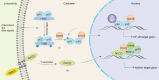
DEAD-box (DDX)5 and DDX17, which belong to the DEAD-box RNA helicase family, are nuclear and cytoplasmic shuttle proteins. These proteins are expressed in most tissues and cells and participate in the regulation of normal physiological functions; their abnormal expression is closely related to tumorigenesis and tumor progression. DDX5/DDX17 participate in almost all processes of RNA metabolism, such as the alternative splicing of mRNA, biogenesis of microRNAs (miRNAs) and ribosomes, degradation of mRNA, interaction with long noncoding RNAs (lncRNAs) and coregulation of transcriptional activity. Moreover, different posttranslational modifications, such as phosphorylation, acetylation, ubiquitination, and sumoylation, endow DDX5/DDX17 with different functions in tumorigenesis and tumor progression. Indeed, DDX5 and DDX17 also interact with multiple key tumor-promoting molecules and participate in tumorigenesis and tumor progression signaling pathways. When DDX5/DDX17 expression or their posttranslational modification is dysregulated, the normal cellular signaling network collapses, leading to many pathological states, including tumorigenesis and tumor development. This review mainly discusses the molecular structure features and biological functions of DDX5/DDX17 and their effects on tumorigenesis and tumor progression, as well as their potential clinical application for tumor treatment.
Keywords: DDX17; DDX5; RNA transcription regulation; posttranslational modification; tumor progression; tumorigenesis.
Copyright © 2022 Xu, Sun, Yan, Cui, Yang, Li, Huang, Dou, Chen, Tang, Lan, Li and Shen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Upregulation of DEAD box helicase 5 and 17 are correlated with the progression and poor prognosis in gliomas.Pathol Res Pract. 2020 Mar;216(3):152828. doi: 10.1016/j.prp.2020.152828. Epub 2020 Jan 20. Pathol Res Pract. 2020. PMID: 32008867
-
Dual role of the ddx5/ddx17 RNA helicases in the control of the pro-migratory NFAT5 transcription factor.Oncogene. 2012 Oct 18;31(42):4536-49. doi: 10.1038/onc.2011.618. Epub 2012 Jan 23. Oncogene. 2012. PMID: 22266867
-
DEAD box RNA helicase 5 is a new pro-viral host factor for Sindbis virus infection.Virol J. 2024 Mar 29;21(1):76. doi: 10.1186/s12985-024-02349-3. Virol J. 2024. PMID: 38553727 Free PMC article.
-
The DDX5/Dbp2 subfamily of DEAD-box RNA helicases.Wiley Interdiscip Rev RNA. 2019 Mar;10(2):e1519. doi: 10.1002/wrna.1519. Epub 2018 Dec 2. Wiley Interdiscip Rev RNA. 2019. PMID: 30506978 Free PMC article. Review.
-
Functions of DEAD box RNA helicases DDX5 and DDX17 in chromatin organization and transcriptional regulation.BMB Rep. 2018 Dec;51(12):613-622. doi: 10.5483/BMBRep.2018.51.12.234. BMB Rep. 2018. PMID: 30293550 Free PMC article. Review.
Cited by
-
Role and therapeutic potential of DEAD-box RNA helicase family in colorectal cancer.Front Oncol. 2023 Oct 27;13:1278282. doi: 10.3389/fonc.2023.1278282. eCollection 2023. Front Oncol. 2023. PMID: 38023215 Free PMC article. Review.
-
Screening and identification of versican as a sensitive biomarker and potential therapeutic target in basal cell carcinoma.Int J Med Sci. 2025 Apr 28;22(10):2488-2501. doi: 10.7150/ijms.105650. eCollection 2025. Int J Med Sci. 2025. PMID: 40386063 Free PMC article.
-
Unraveling the ecological landscape of mast cells in esophageal cancer through single-cell RNA sequencing.Front Immunol. 2024 Oct 4;15:1470449. doi: 10.3389/fimmu.2024.1470449. eCollection 2024. Front Immunol. 2024. PMID: 39430754 Free PMC article.
-
Prognostic and immunological landscape of DDX17 in pan-cancer analysis: a comprehensive study.Discov Oncol. 2025 Jun 17;16(1):1132. doi: 10.1007/s12672-025-02955-9. Discov Oncol. 2025. PMID: 40526173 Free PMC article.
-
Mapping adipocyte interactome networks by HaloTag-enrichment-mass spectrometry.Biol Methods Protoc. 2024 May 29;9(1):bpae039. doi: 10.1093/biomethods/bpae039. eCollection 2024. Biol Methods Protoc. 2024. PMID: 38884001 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

