BTK, the new kid on the (oncology) block?
- PMID: 35992808
- PMCID: PMC9386470
- DOI: 10.3389/fonc.2022.944538
BTK, the new kid on the (oncology) block?
Abstract
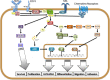
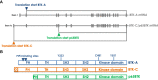
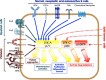
In the last decade data piled up indicating that BTK - for twenty years considered as a "private matter" of bone marrow-derived cells - it is expressed and plays important and different roles also outside of the hematopoietic compartment and, most notably, in tumor cells. Initial evidence that BTK plays a critical role in B cell-derived malignancies prompted the chase for specific inhibitors, the forefather of which entered the clinic in a record time and paved the way for an ever increasing number of new molecules to be trialed. The growing interests in BTK also led to the discovery that, in solid tumors, two novel isoforms are mainly expressed and actionable liabilities for target therapy. Remarkably, the different isoforms appear to be involved in different signaling pathways which will have to be attentively specified in order to define the area of therapeutic intervention. In this perspective we briefly summarize the progress made in the last decade in studying BTK and its isoforms in cancer cells and define the open questions to be addressed in order to get the most benefits from its targeting for therapeutic purposes.
Keywords: B-cell leukemia; BTK; BTK-A; BTK-C; p65BTK; solid tumors.
Copyright © 2022 Grassilli, Cerrito and Lavitrano.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Satterthwaite AB, Willis F, Kanchanastit P, Fruman D, Cantley LC, Helgason CD, et al. . A sensitized genetic system for the analysis of murine b lymphocyte signal transduction pathways dependent on bruton’s tyrosine kinase. Proc Natl Acad Sci USA (2000) 97:6687–92. doi: 10.1073/pnas.110146697 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

