PD-L1 expression, tumor mutational burden, and immune cell infiltration in non-small cell lung cancer patients with epithelial growth factor receptor mutations
- PMID: 35992815
- PMCID: PMC9389166
- DOI: 10.3389/fonc.2022.922899
PD-L1 expression, tumor mutational burden, and immune cell infiltration in non-small cell lung cancer patients with epithelial growth factor receptor mutations
Abstract
Background: Immunotherapy using programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) inhibitors seems less effective in non-small cell lung cancer (NSCLC) patients with epithelial growth factor receptor (EGFR) mutations. Varied responses to PD-1/PD-L1 inhibitors have recently been observed in NSCLC patients harboring different types of EGFR mutations. Some EGFR-mutated NSCLC patients may benefit from PD-1/PD-L1 inhibitors. At present, PD-L1 expression, tumor mutational burden (TMB), and tumor immune microenvironment (TIME) are biomarkers for predicting the efficacy of PD-1/PD-L1 inhibitors in NSCLC patients. We retrospectively evaluated PD-L1 expression, TMB, and immune cell infiltration in NSCLC patients with EGFR mutation subtypes.
Methods: PD-L1 expression, TMB, and the abundance of immune cell infiltration in NSCLC patients were evaluated in public databases and clinical samples. TMB was detected using the NGS technique, PD-L1 was detected using immunohistochemistry, and the abundance of immune cell infiltration in NSCLC samples was detected using multiple immunohistochemistry.
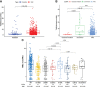
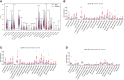
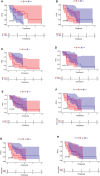
Results: PD-L1 expression and TMB were lower in EGFR-mutated NSCLCs than in wild-type patients. Differences in the abundance of immune cell infiltration were also observed between EGFR-mutated and wild-type NSCLC. The expression of PD-L1, TMB, and abundance of immune cell infiltration were different in patients harboring different subtypes of EGFR mutations. Patients with uncommon EGFR mutations, especially the G719X mutation, showed higher TMB and expressions of PD-L1 than classical EGFR mutations. M1 macrophages were higher in uncommon EGFR mutations than classical EGFR mutations.
Conclusions: The expression of PD-L1 and TMB in uncommon EGFR-mutated NSCLCs, especially the G719X mutation, were higher than those for classical EGFR-mutated NSCLCs and similar to EGFR wild-type. The abundance of immune cell infiltration in uncommon EGFR-mutated NSCLCs was similar to that in EGFR wild-type. Our findings suggest that uncommon EGFR-mutated NSCLCs may benefit from PD-1/PD-L1 inhibitors.
Keywords: Epidermal growth factor receptor; Non-small cell lung cancer; Programmed death-ligand 1; Tumor immune microenvironment; Tumor mutational burden.
Copyright © 2022 Ma, Jiao, Huo, Li, Fang, Zhao, Liu, Han, Xi, Wang and Shang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Zhou C, Wu Y-L, Chen G, Feng J, Liu X-Q, Wang C, et al. . Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol (2011) 12:735–42. doi: 10.1016/S1470-2045(11)70184-X - DOI - PubMed
-
- Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. . Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol (2012) 13:239–46. doi: 10.1016/S1470-2045(11)70393-X - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

