Selection of the Optimal Method for Creating Various Forms of Biocompatible Xenodermal Materials
- PMID: 35993001
- PMCID: PMC9376758
- DOI: 10.17691/stm2022.14.1.04
Selection of the Optimal Method for Creating Various Forms of Biocompatible Xenodermal Materials
Abstract
The aim of the study was to select the optimal method for creating surgical porcine dermis-based biomaterials and to assess their biological safety.
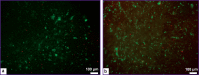
Materials and methods: To create xenodermal biomaterials, the native skin of a 4-month-old Landrace pig was used. The porcine dermis was processed with saline (protocol No.1), peroxide-alkaline (protocol No.2), and alkaline (protocol No.3) solutions. The obtained samples were stained with hematoxylin-eosin and a DAPI fluorescent dye. Quantitative DNA analysis and assessment of cytotoxicity by the LIVE/DEAD assay were also performed. Samples were implanted/injected subcutaneously to 6-month-old male Wistar rats (n=30) weighing 260±20 g and explanted on day 14 of the experiment. Histological sections were stained with hematoxylin-eosin. Computer morphometry was performed using GraphPad Prism v. 6.04.
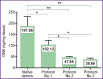
Results: Samples of surgical materials obtained according to the three protocols had different physical characteristics: dermis treated according to protocol No.1 was dense and white in color after processing; samples processed by protocol No.2 were transparent and dense, and samples treated according to protocol No.3 had transparent gel-like structures. Histological analysis has shown oxyphilicity and extracellular matrix structure loss in all samples, and DAPI staining has revealed the destruction of cell nuclei. Nevertheless, DNA amount in the samples processed according to protocol No.1 did not meet the established quality criterion for decellularization (50 ng/mg dry weight). Further cytotoxicity assessment in vitro and in vivo was carried out only for samples fabricated according to protocols No.2 and No.3. According to the LIVE/DEAD analysis, both samples were not cytotoxic. On day 14 after the subcutaneous sample implantation, no signs of suppuration and immune rejection were found in the animals.
Conclusion: To obtain surgical materials in the form of bioplastic coatings, it is recommended to use alkaline-peroxide treatment of the dermis, while hydrogel coatings are produced by alkaline hydrolysis.
Keywords: alkaline hydrolysis; decellularization; dermis; surgical biomaterials; xenodermal biomaterials.
Conflict of interest statement
Conflicts of interest. The authors declare no evident or potential conflicts of interest associated with the publication of this article.
Figures





References
-
- Ushmarov D.I., Gumenyuk S.E., Gumenyuk A.S., Gayvoronskaya T.V., Karablina S.Y., Pomortsev А.V., Sotnichenko A.S., Melkonyan K.I., Grigoriev T.Е. Comparative evaluation of chitosan-based multifunctional wound dressings: a multistage randomised controlled experimental trial. Kubanskii nauchnyi meditsinskii vestnik. 2021;28(3):78–96. doi: 10.25207/1608-6228-2021-28-3-78-96. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
