Exosome Derived from Human Neural Stem Cells Improves Motor Activity and Neurogenesis in a Traumatic Brain Injury Model
- PMID: 35993050
- PMCID: PMC9391191
- DOI: 10.1155/2022/6409346
Exosome Derived from Human Neural Stem Cells Improves Motor Activity and Neurogenesis in a Traumatic Brain Injury Model
Abstract
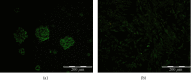
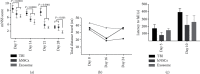
Traumatic brain injury (TBI) is a leading cause of mortality and long-lasting disability globally. Although novel treatment options have been investigated, no effective therapeutic opportunities for TBI exist. Accumulating studies demonstrated that the paracrine mechanisms of stem cells may allow them to orchestrate regenerative processes after TBI. So far, very little attention has been paid to the beneficial effects of human neural stem cells (hNSCs) in comparison to their exosomes as a paracrine mechanism. This study is aimed at comparing the effect of hNSCs with their exosomes in a TBI model. For in vitro assessments, we cultured hNSCs using the neurosphere method and isolated hNSC-derived exosomes from culture supernatants. For in vivo experiments, male rats were divided into three groups (n = 8/group): TBI group: rats were subjected to a unilateral mild cortical impact; hNSC group: rats received a single intralesional injection of 2 × 106 hNSCs after TBI; and exosome group: rats received a single intralesional injection of 63 μg protein of hNSC-derived exosomes after TBI. Neurological assessments, neuroinflammation, and neurogenesis were performed at the predetermined time points after TBI. Our results indicated that the administration of exosomes improved the neurobehavioral performance measured by the modified neurological severity score (mNSS) on day 28 after TBI. Furthermore, exosomes inhibited the expression of reactive astrocytes as a key regulator of neuroinflammation marked by GFAP at the protein level, while enhancing the expression of Doublecortin (DCX) as a neurogenesis marker at the mRNA level. On the other hand, we observed that the expression of stemness markers (SOX2 and Nestin) was elevated in the hNSC group compared to the exosome and TBI groups. To sum up, our results demonstrated that the superior effects of exosomes versus parent hNSCs could be mediated by improving mNSS score and increasing DCX in TBI. Considerably, more work will need to be done to determine the beneficial effects of exosomes versus parent cells in the context of TBI.
Copyright © 2022 Mahsa Abedi et al.
Conflict of interest statement
All authors declared that there is no conflict of interest.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

