CTCF shapes chromatin structure and gene expression in health and disease
- PMID: 35993175
- PMCID: PMC9442299
- DOI: 10.15252/embr.202255146
CTCF shapes chromatin structure and gene expression in health and disease
Abstract
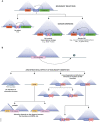
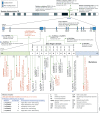
CCCTC-binding factor (CTCF) is an eleven zinc finger (ZF), multivalent transcriptional regulator, that recognizes numerous motifs thanks to the deployment of distinct combinations of its ZFs. The great majority of the ~50,000 genomic locations bound by the CTCF protein in a given cell type is intergenic, and a fraction of these sites overlaps with transcriptional enhancers. Furthermore, a proportion of the regions bound by CTCF intersect genes and promoters. This suggests multiple ways in which CTCF may impact gene expression. At promoters, CTCF can directly affect transcription. At more distal sites, CTCF may orchestrate interactions between regulatory elements and help separate eu- and heterochromatic areas in the genome, exerting a chromatin barrier function. In this review, we outline how CTCF contributes to the regulation of the three-dimensional structure of chromatin and the formation of chromatin domains. We discuss how CTCF binding and architectural functions are regulated. We examine the literature implicating CTCF in controlling gene expression in development and disease both by acting as an insulator and a factor facilitating regulatory elements to efficiently interact with each other in the nuclear space.
Keywords: CTCF; chromatin structure; enhancer; insulator; regulation of gene expression.
© 2022 The Authors. Published under the terms of the CC BY 4.0 license.
Figures

References
-
- Akhtar W, de Jong J, Pindyurin AV, Pagie L, Meuleman W, de Ridder J, Berns A, Wessels LFA, van Lohuizen M, van Steensel B (2013) Chromatin position effects assayed by thousands of reporters integrated in parallel. Cell 154: 914–927 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

