Insights into mechanisms of graft-versus-host disease through humanised mouse models
- PMID: 35993192
- PMCID: PMC9446388
- DOI: 10.1042/BSR20211986
Insights into mechanisms of graft-versus-host disease through humanised mouse models
Abstract
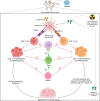
Graft-versus-host disease (GVHD) is a major complication that occurs following allogeneic haematopoietic stem cell transplantation (HSCT) for the treatment of haematological cancers and other blood-related disorders. GVHD is an inflammatory disorder, where the transplanted donor immune cells can mediate an immune response against the recipient and attack host tissues. Despite over 60 years of research, broad-range immune suppression is still used to prevent or treat GVHD, leading to an increased risk of cancer relapse and infection. Therefore, further insights into the disease mechanisms and development of predictive and prognostic biomarkers are key to improving outcomes and reducing GVHD development following allogeneic HSCT. An important preclinical tool to examine the pathophysiology of GVHD and to understand the key mechanisms that lead to GVHD development are preclinical humanised mouse models. Such models of GVHD are now well-established and can provide valuable insights into disease development. This review will focus on models where human peripheral blood mononuclear cells are injected into immune-deficient non-obese diabetic (NOD)-scid-interleukin-2(IL-2)Rγ mutant (NOD-scid-IL2Rγnull) mice. Humanised mouse models of GVHD can mimic the clinical setting for GVHD development, with disease progression and tissues impacted like that observed in humans. This review will highlight key findings from preclinical humanised mouse models regarding the role of donor human immune cells, the function of cytokines and cell signalling molecules and their impact on specific target tissues and GVHD development. Further, specific therapeutic strategies tested in these preclinical models reveal key molecular pathways important in reducing the burden of GVHD following allogeneic HSCT.
Keywords: Allogeneic haematopoietic stem cell transplantation; Antigen presenting cell; Graft-versus-host disease; Inflammation; Lymphocyte; Xenogeneic graft-versus-host disease.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

References
-
- Niederwieser D., Baldomero H., Bazuaye N., Bupp C., Chaudhri N., Corbacioglu S.et al. (2021) One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica 107, 1045–1053 10.3324/haematol.2021.279189 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

