Portable electroanalytical nucleic acid amplification tests using printed circuit boards and open-source electronics
- PMID: 35993403
- PMCID: PMC9511072
- DOI: 10.1039/d2an00923d
Portable electroanalytical nucleic acid amplification tests using printed circuit boards and open-source electronics
Abstract
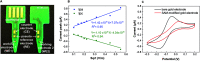
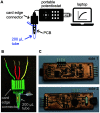
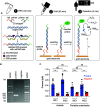
The realization of electrochemical nucleic acid amplification tests (NAATs) at the point of care (POC) is highly desirable, but it remains a challenge given their high cost and lack of true portability/miniaturization. Here we show that mass-produced, industrial standardized, printed circuit boards (PCBs) can be repurposed to act as near-zero cost electrodes for self-assembled monolayer-based DNA biosensing, and further integration with a custom-designed and low-cost portable potentiostat. To show the analytical capability of this system, we developed a NAAT using isothermal recombinase polymerase amplification, bypassing the need of thermal cyclers, followed by an electrochemical readout relying on a sandwich hybridization assay. We used our sensor and device for analytical detection of the toxic microalgae Ostreopsis cf. ovata as a proof of concept. This work shows the potential of PCBs and open-source electronics to be used as powerful POC DNA biosensors at a low-cost.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Toward Continuous Molecular Testing Using Gold-Coated Threads as Multi-Target Electrochemical Biosensors.Biosensors (Basel). 2023 Aug 25;13(9):844. doi: 10.3390/bios13090844. Biosensors (Basel). 2023. PMID: 37754078 Free PMC article.
-
Electroanalytical Paper-Based Nucleic Acid Amplification Biosensors with Integrated Thread Electrodes.Anal Chem. 2021 Oct 26;93(42):14187-14195. doi: 10.1021/acs.analchem.1c02900. Epub 2021 Oct 14. Anal Chem. 2021. PMID: 34648274 Free PMC article.
-
Woven Electroanalytical Biosensor for Nucleic Acid Amplification Tests.Adv Healthc Mater. 2021 Jun;10(11):e2100034. doi: 10.1002/adhm.202100034. Epub 2021 Apr 30. Adv Healthc Mater. 2021. PMID: 33930257
-
Advances in nucleic acid amplification techniques (NAATs): COVID-19 point-of-care diagnostics as an example.Biosens Bioelectron. 2022 Jun 15;206:114109. doi: 10.1016/j.bios.2022.114109. Epub 2022 Feb 26. Biosens Bioelectron. 2022. PMID: 35245867 Review.
-
Biosensors Based on Isothermal DNA Amplification for Bacterial Detection in Food Safety and Environmental Monitoring.Sensors (Basel). 2021 Jan 16;21(2):602. doi: 10.3390/s21020602. Sensors (Basel). 2021. PMID: 33467078 Free PMC article. Review.
Cited by
-
Low-cost portable sensor for rapid and sensitive detection of Pb2+ ions using capacitance sensing integrated with microfluidic enrichment.Mikrochim Acta. 2024 Oct 30;191(11):718. doi: 10.1007/s00604-024-06798-z. Mikrochim Acta. 2024. PMID: 39477888
-
Toward Continuous Molecular Testing Using Gold-Coated Threads as Multi-Target Electrochemical Biosensors.Biosensors (Basel). 2023 Aug 25;13(9):844. doi: 10.3390/bios13090844. Biosensors (Basel). 2023. PMID: 37754078 Free PMC article.
-
Recent advances in bio-microsystem integration and Lab-on-PCB technology.Microsyst Nanoeng. 2025 May 8;11(1):78. doi: 10.1038/s41378-025-00940-4. Microsyst Nanoeng. 2025. PMID: 40335457 Free PMC article. Review.
References
-
- Zhao Y. X. Zuo X. L. Li Q. Chen F. Chen Y. R. Deng J. Q. Han D. Hao C. L. Huang F. J. Huang Y. Y. Ke G. L. Kuang H. Li F. Li J. Li M. Li N. Lin Z. Y. Liu D. B. Liu J. W. Liu L. B. Liu X. G. Lu C. H. Luo F. Mao X. H. Sun J. S. Tang B. Wang F. Wang J. B. Wang L. H. Wang S. Wu L. L. Wu Z. S. Xia F. Xu C. L. Yang Y. Yuan B. F. Yuan Q. Zhang C. Zhu Z. Yang C. Y. Zhang X. B. Yang H. H. Tan W. H. Fan C. H. Sci. China: Chem. 2021;64:171–203. doi: 10.1007/s11426-020-9864-7. - DOI - PMC - PubMed
-
- Corman V. M. Landt O. Kaiser M. Molenkamp R. Meijer A. Chu D. K. W. Bleicker T. Brunink S. Schneider J. Schmidt M. L. Mulders D. Haagmans B. L. van der Veer B. van den Brink S. Wijsman L. Goderski G. Romette J. L. Ellis J. Zambon M. Peiris M. Goossens H. Reusken C. Koopmans M. P. G. Drosten C. Eurosurveillance. 2020;25:23–30.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

