Gestational Benzo[a]pyrene Exposure Destroys F1 Ovarian Germ Cells Through Mitochondrial Apoptosis Pathway and Diminishes Surviving Oocyte Quality
- PMID: 35993611
- PMCID: PMC9960072
- DOI: 10.1093/toxsci/kfac086
Gestational Benzo[a]pyrene Exposure Destroys F1 Ovarian Germ Cells Through Mitochondrial Apoptosis Pathway and Diminishes Surviving Oocyte Quality
Abstract
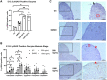
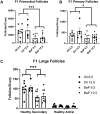
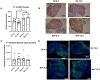
Polycyclic aromatic hydrocarbons, including benzo[a]pyrene (BaP), are products of incomplete combustion. In female mouse embryos primordial germ cells proliferate before and after arriving at the gonadal ridge around embryonic (E) 10 and begin entering meiosis at E13.5. Now oocytes, they arrest in the first meiotic prophase beginning at E17.5. We previously reported dose-dependent depletion of ovarian follicles in female mice exposed to 2 or 10 mg/kg-day BaP E6.5-15.5. We hypothesized that embryonic ovaries are more sensitive to gestational BaP exposure during the mitotic developmental window, and that this exposure results in persistent oxidative stress in ovaries and oocytes of exposed F1 female offspring. We orally dosed timed-pregnant female mice with 0 or 2 mg/kg-day BaP in oil from E6.5-11.5 (mitotic window) or E12.5-17.5 (meiotic window). Cultured E13.5 ovaries were utilized to investigate the mechanism of BaP-induced germ cell death. We observed statistically significant follicle depletion and increased ovarian lipid peroxidation in F1 pubertal ovaries following BaP exposure during either prenatal window. Culture of E13.5 ovaries with BaP induced germ cell DNA damage and release of cytochrome c from the mitochondria in oocytes, confirming that BaP exposure induced apoptosis via the mitochondrial pathway. Mitochondrial membrane potential, oocyte lipid droplet (LD) volume, and mitochondrial-LD colocalization were decreased and mitochondrial superoxide levels were increased in the MII oocytes of F1 females exposed gestationally to BaP. Results demonstrate similar sensitivity to germ cell depletion and persistent oxidative stress in F1 ovaries and oocytes following gestational BaP exposure during mitotic or meiotic windows.
Keywords: benzo[a]pyrene; lipid droplets; mitochondria; oocyte; oxidative stress; polycyclic aromatic hydrocarbons.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures





Similar articles
-
Prenatal exposure to benzo[a]pyrene depletes ovarian reserve and masculinizes embryonic ovarian germ cell transcriptome transgenerationally.Sci Rep. 2023 May 29;13(1):8671. doi: 10.1038/s41598-023-35494-w. Sci Rep. 2023. PMID: 37248279 Free PMC article.
-
Glutathione deficiency sensitizes cultured embryonic mouse ovaries to benzo[a]pyrene-induced germ cell apoptosis.Toxicol Appl Pharmacol. 2018 Aug 1;352:38-45. doi: 10.1016/j.taap.2018.05.024. Epub 2018 May 22. Toxicol Appl Pharmacol. 2018. PMID: 29800640 Free PMC article.
-
Maternal benzo[a]pyrene exposure is correlated with the meiotic arrest and quality deterioration of offspring oocytes in mice.Reprod Toxicol. 2020 Apr;93:10-18. doi: 10.1016/j.reprotox.2019.12.003. Epub 2019 Dec 23. Reprod Toxicol. 2020. PMID: 31874190
-
Establishment of oocyte population in the fetal ovary: primordial germ cell proliferation and oocyte programmed cell death.Reprod Biomed Online. 2005 Feb;10(2):182-91. doi: 10.1016/s1472-6483(10)60939-x. Reprod Biomed Online. 2005. PMID: 15823221 Review.
-
Dynamic changes of DNA methylation induced by benzo(a)pyrene in cancer.Genes Environ. 2023 Jul 1;45(1):21. doi: 10.1186/s41021-023-00278-1. Genes Environ. 2023. PMID: 37391844 Free PMC article. Review.
Cited by
-
Fertility loss: negative effects of environmental toxicants on oogenesis.Front Physiol. 2023 Aug 4;14:1219045. doi: 10.3389/fphys.2023.1219045. eCollection 2023. Front Physiol. 2023. PMID: 37601637 Free PMC article. Review.
-
Prenatal exposure to benzo[a]pyrene depletes ovarian reserve and masculinizes embryonic ovarian germ cell transcriptome transgenerationally.Sci Rep. 2023 May 29;13(1):8671. doi: 10.1038/s41598-023-35494-w. Sci Rep. 2023. PMID: 37248279 Free PMC article.
References
-
- Adhikari D., Lee I., Yuen W. S., Carroll J. (2022). Oocyte mitochondria—Key regulators of oocyte function and potential therapeutic targets for improving fertility. Biol. Reprod. 106, 366–377. - PubMed
-
- Aizawa R., Ibayashi M., Tatsumi T., Yamamoto A., Kokubo T., Miyasaka N., Sato K., Ikeda S., Minami N., Tsukamoto S. (2019). Synthesis and maintenance of lipid droplets are essential for mouse preimplantation embryonic development. Development 146, 1–11. - PubMed
-
- Benador I. Y., Veliova M., Mahdaviani K., Petcherski A., Wikstrom J. D., Assali E. A., Acín-Pérez R., Shum M., Oliveira M. F., Cinti S., et al. (2018). Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metab. 27, 869–885. - PMC - PubMed
-
- Bianchi S., Macchiarelli G., Micara G., Linari A., Boninsegna C., Aragona C., Rossi G., Cecconi S., Nottola S. A. (2015). Ultrastructural markers of quality are impaired in human metaphase II aged oocytes: A comparison between reproductive and in vitro aging. J. Assist. Reprod. Genet. 32, 1343–1358. - PMC - PubMed

