Laboratory Plasticware Induces Expression of a Bacterial Virulence Factor
- PMID: 35993764
- PMCID: PMC9599451
- DOI: 10.1128/msphere.00311-22
Laboratory Plasticware Induces Expression of a Bacterial Virulence Factor
Abstract
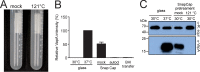
Pollution with microplastic has become a prime environmental concern. The various ways in which human-made polymers and microorganisms interact are little understood, and this is particularly true for microplastic and pathogenic microorganisms. Previous reports demonstrated that expression of central virulence-associated protein A (VapA) of the pathogenic bacterium Rhodococcus equi is shut off at 30°C, whereas it is strongly expressed at 37°C, a temperature which may serve as an intrahost cue. Here, we show that cultivation at 30°C in disposable plastic tubes increases mRNA levels of vapA 70-fold compared to growth in conventional glass tubes. Strong expression of vapA in plastic tubes does not seem to be caused by a compound leaching from plastic but rather by tube surface properties. Expression stimulation during growth in plastic is regulated by the R. equi transcription regulators VirR and VirS, indicating that plastic-induced vapA expression is (co)regulated through the canonical vapA expression pathway. Our observations have important implications for the future analysis and assessment of environmental microplastic contaminations in that they show that, in principle, contact of pathogens with environmental plastic can increase their virulence. IMPORTANCE Millions of tons small plastic pieces (microplastic) find their way into the environment every year. They pose digestive and toxicity problems to various life forms in soil, freshwater, and seawater. Additionally, microplastic offers an opportunity for microorganisms to attach and to become an important part of a "plastisphere community." The significance of our study lies in the documentation of a sharp increase in production of a central virulence factor by a bacterial pathogen when the bacterium is in touch with certain makes of plastic. Although this feature may not reflect an increased health risk in case of this particular soilborne pathogen, our data disclose a new facet of how microplastics can endanger life.
Keywords: Rhodococcus; gene expression; microplastic; pathogen; pollution; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
The LysR-type transcriptional regulator VirR is required for expression of the virulence gene vapA of Rhodococcus equi ATCC 33701.J Bacteriol. 2004 Sep;186(17):5576-84. doi: 10.1128/JB.186.17.5576-5584.2004. J Bacteriol. 2004. PMID: 15317761 Free PMC article.
-
VirS, an OmpR/PhoB subfamily response regulator, is required for activation of vapA gene expression in Rhodococcus equi.BMC Microbiol. 2014 Oct 3;14:243. doi: 10.1186/s12866-014-0243-1. BMC Microbiol. 2014. PMID: 25281192 Free PMC article.
-
The vapA co-expressed virulence plasmid gene vcgB (orf10) of the intracellular actinomycete Rhodococcus equi.Microbiology (Reading). 2011 Aug;157(Pt 8):2357-2368. doi: 10.1099/mic.0.049759-0. Epub 2011 May 12. Microbiology (Reading). 2011. PMID: 21565932
-
Pathogenesis and virulence of Rhodococcus equi.Vet Microbiol. 1997 Jun 16;56(3-4):257-68. doi: 10.1016/s0378-1135(97)00094-1. Vet Microbiol. 1997. PMID: 9226840 Review.
-
Microplastic contaminants in the aqueous environment, fate, toxicity consequences, and remediation strategies.Environ Res. 2021 Sep;200:111762. doi: 10.1016/j.envres.2021.111762. Epub 2021 Jul 24. Environ Res. 2021. PMID: 34310963 Review.
Cited by
-
Microplastic diversity increases the abundance of antibiotic resistance genes in soil.Nat Commun. 2024 Nov 12;15(1):9788. doi: 10.1038/s41467-024-54237-7. Nat Commun. 2024. PMID: 39532872 Free PMC article.
References
-
- Gundelly P, Suzuki Y, Ribes JA, Thornton A. 2016. Differences in Rhodococcus equi infections based on immune status and antibiotic susceptibility of clinical isolates in a case series of 12 patients and cases in the literature. Biomed Res Int 2016:2737295. doi:10.1155/2016/2737295. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

