Cryo-EM structure of the Smc5/6 holo-complex
- PMID: 35993814
- PMCID: PMC9458440
- DOI: 10.1093/nar/gkac692
Cryo-EM structure of the Smc5/6 holo-complex
Abstract
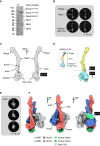
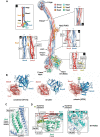
The Smc5/6 complex plays an essential role in the resolution of recombination intermediates formed during mitosis or meiosis, or as a result of the cellular response to replication stress. It also functions as a restriction factor preventing viral replication. Here, we report the cryogenic EM (cryo-EM) structure of the six-subunit budding yeast Smc5/6 holo-complex, reconstituted from recombinant proteins expressed in insect cells - providing both an architectural overview of the entire complex and an understanding of how the Nse1/3/4 subcomplex binds to the hetero-dimeric SMC protein core. In addition, we demonstrate that a region within the head domain of Smc5, equivalent to the 'W-loop' of Smc4 or 'F-loop' of Smc1, mediates an important interaction with Nse1. Notably, mutations that alter the surface-charge profile of the region of Nse1 which accepts the Smc5-loop, lead to a slow-growth phenotype and a global reduction in the chromatin-associated fraction of the Smc5/6 complex, as judged by single molecule localisation microscopy experiments in live yeast. Moreover, when taken together, our data indicates functional equivalence between the structurally unrelated KITE and HAWK accessory subunits associated with SMC complexes.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
-
- Matityahu A., Onn I.. Hit the brakes - a new perspective on the loop extrusion mechanism of cohesin and other SMC complexes. J. Cell Sci. 2021; 134:jcs247577. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

