Metabolism of 3-Chlorobiphenyl (PCB 2) in a Human-Relevant Cell Line: Evidence of Dechlorinated Metabolites
- PMID: 35994059
- PMCID: PMC9573771
- DOI: 10.1021/acs.est.2c03687
Metabolism of 3-Chlorobiphenyl (PCB 2) in a Human-Relevant Cell Line: Evidence of Dechlorinated Metabolites
Abstract
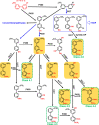
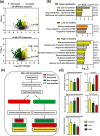
Lower chlorinated polychlorinated biphenyls (LC-PCBs) and their metabolites make up a class of environmental pollutants implicated in a range of adverse outcomes in humans; however, the metabolism of LC-PCBs in human models has received little attention. Here we characterize the metabolism of PCB 2 (3-chlorobiphenyl), an environmentally relevant LC-PCB congener, in HepG2 cells with in silico prediction and nontarget high-resolution mass spectrometry. Twenty PCB 2 metabolites belonging to 13 metabolite classes, including five dechlorinated metabolite classes, were identified in the cell culture media from HepG2 cells exposed for 24 h to 10 μM or 3.6 nM PCB 2. The PCB 2 metabolite profiles differed from the monochlorinated metabolite profiles identified in samples from an earlier study with PCB 11 (3,3'-dichlorobiphenyl) under identical experimental conditions. A dechlorinated dihydroxylated metabolite was also detected in human liver microsomal incubations with monohydroxylated PCB 2 metabolites but not PCB 2. These findings demonstrate that the metabolism of LC-PCBs in human-relevant models involves the formation of dechlorination products. In addition, untargeted metabolomic analyses revealed an altered bile acid biosynthesis in HepG2 cells. Our results indicate the need to study the disposition and toxicity of complex PCB 2 metabolites, including novel dechlorinated metabolites, in human-relevant models.
Keywords: ADMET predictor; HepG2 cells; MetaDrug; Nt-HRMS; PCB 2 metabolites; dechlorination; untargeted metabolomics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
3,3'-Dichlorobiphenyl Is Metabolized to a Complex Mixture of Oxidative Metabolites, Including Novel Methoxylated Metabolites, by HepG2 Cells.Environ Sci Technol. 2020 Oct 6;54(19):12345-12357. doi: 10.1021/acs.est.0c03476. Epub 2020 Sep 23. Environ Sci Technol. 2020. PMID: 32910851 Free PMC article.
-
Characterization of the Metabolic Pathways of 4-Chlorobiphenyl (PCB3) in HepG2 Cells Using the Metabolite Profiles of Its Hydroxylated Metabolites.Environ Sci Technol. 2021 Jul 6;55(13):9052-9062. doi: 10.1021/acs.est.1c01076. Epub 2021 Jun 14. Environ Sci Technol. 2021. PMID: 34125531 Free PMC article.
-
Nontarget analysis reveals gut microbiome-dependent differences in the fecal PCB metabolite profiles of germ-free and conventional mice.Environ Pollut. 2021 Jan 1;268(Pt A):115726. doi: 10.1016/j.envpol.2020.115726. Epub 2020 Sep 25. Environ Pollut. 2021. PMID: 33032095 Free PMC article.
-
Occurrence and distribution of PCB metabolites in blood and their potential health effects in humans: a review.Environ Sci Pollut Res Int. 2014 Oct;21(20):11951-72. doi: 10.1007/s11356-014-3136-9. Epub 2014 Jun 19. Environ Sci Pollut Res Int. 2014. PMID: 24943885 Review.
-
Microbial reductive dechlorination of PCBs.Biodegradation. 1993-1994;4(4):231-40. doi: 10.1007/BF00695971. Biodegradation. 1993. PMID: 7764920 Review.
Cited by
-
Elucidating the Metabolism of Chiral PCB95 in Wildtype and Transgenic Mouse Models with Altered Cytochrome P450 Enzymes Using Intestinal Content Screening.Chem Res Toxicol. 2024 Dec 16;37(12):1989-2002. doi: 10.1021/acs.chemrestox.4c00350. Epub 2024 Nov 19. Chem Res Toxicol. 2024. PMID: 39561283 Free PMC article.
-
Disposition and metabolomic effects of 2,2',5,5'-tetrachlorobiphenyl in female rats following intraperitoneal exposure.Environ Toxicol Pharmacol. 2023 Sep;102:104245. doi: 10.1016/j.etap.2023.104245. Epub 2023 Aug 11. Environ Toxicol Pharmacol. 2023. PMID: 37572994 Free PMC article.
-
Complex roles for sulfation in the toxicities of polychlorinated biphenyls.Crit Rev Toxicol. 2024 Feb;54(2):92-122. doi: 10.1080/10408444.2024.2311270. Epub 2024 Feb 16. Crit Rev Toxicol. 2024. PMID: 38363552 Free PMC article. Review.
-
Partial dechlorination of 2,4,4'-trichlorobiphenyl (PCB 28) mediated by recombinant human CYP1A2.Arch Toxicol. 2024 Jan;98(1):159-163. doi: 10.1007/s00204-023-03621-1. Epub 2023 Nov 2. Arch Toxicol. 2024. PMID: 37917334 Free PMC article. No abstract available.
-
Use of a polymeric implant system to assess the neurotoxicity of subacute exposure to 2,2',5,5'-tetrachlorobiphenyl-4-ol, a human metabolite of PCB 52, in male adolescent rats.Toxicology. 2023 Dec;500:153677. doi: 10.1016/j.tox.2023.153677. Epub 2023 Nov 22. Toxicology. 2023. PMID: 37995827 Free PMC article.
References
-
- Sari M. F.; Esen F.; Cordova Del Aguila D. A.; Kurt Karakus P. B. Passive sampler derived polychlorinated biphenyls (PCBs) in indoor and outdoor air in Bursa, Turkey: Levels and an assessment of human exposure via inhalation. Atmos. Pollut. Res. 2020, 11, 71–80. 10.1016/j.apr.2020.03.001. - DOI
-
- Ranjbar Jafarabadi A.; Riyahi Bakhtiari A.; Mitra S.; Maisano M.; Cappello T.; Jadot C. First polychlorinated biphenyls (PCBs) monitoring in seawater, surface sediments and marine fish communities of the Persian Gulf: Distribution, levels, congener profile and health risk assessment. Environ. Pollut. 2019, 253, 78–88. 10.1016/j.envpol.2019.07.023. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

