Kidney biopsy diagnosis in childhood in the Norwegian Kidney Biopsy Registry and the long-term risk of kidney replacement therapy: a 25-year follow-up
- PMID: 35994104
- PMCID: PMC9925570
- DOI: 10.1007/s00467-022-05706-y
Kidney biopsy diagnosis in childhood in the Norwegian Kidney Biopsy Registry and the long-term risk of kidney replacement therapy: a 25-year follow-up
Abstract
Background: There is scarce information on biopsy-verified kidney disease in childhood and its progression to chronic kidney disease stage 5 (CKD 5). This study aims to review biopsy findings in children, and to investigate risk of kidney replacement therapy (KRT).
Methods: We conducted a retrospective long-term follow-up study of children included in the Norwegian Kidney Biopsy Registry (NKBR) and in the Norwegian Renal Registry (NRR) from 1988 to 2021.
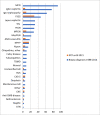
Results: In total, 575 children with a median (interquartile range, IQR) age of 10.7 (6.1 to 14.1) years were included, and median follow-up time (IQR) after kidney biopsy was 14.3 (range 8.9 to 21.6) years. The most common biopsy diagnoses were minimal change disease (MCD; n = 92), IgA vasculitis nephritis (IgAVN; n = 76), IgA nephropathy (n = 63), and focal and segmental glomerulosclerosis (FSGS; n = 47). In total, 118 (20.5%) of the biopsied children reached CKD 5, median (IQR) time to KRT 2.3 years (7 months to 8.4 years). Most frequently, nephronophthisis (NPHP; n = 16), FSGS (n = 30), IgA nephropathy (n = 9), and membranoproliferative glomerulonephritis (MPGN; n = 9) led to KRT.
Conclusions: The risk of KRT after a kidney biopsy diagnosis is highly dependent on the diagnosis. None of the children with MCD commenced KRT, while 63.8% with FSGS and 100% with NPHP reached KRT. Combining data from kidney biopsy registries with registries on KRT allows for detailed information concerning the risk for later CKD 5 after biopsy-verified kidney disease in childhood. A higher resolution version of the Graphical abstract is available as Supplementary information.
Keywords: Children; Chronic kidney disease stage 5; Kidney biopsy; Kidney replacement therapy; National registries.
© 2022. The Author(s).
Conflict of interest statement
Ann Christin Gjerstad, Rannveig Skrunes, Camilla Tøndel, Henrik Døllner, Clara Hammarstrøm, and Anna Kristina Bjerre declare no conflicts of interest. Anders Åsberg’s institution(s) received payment for lectures to Sandoz and Astellas Pharma. Sabine Leh received lecture fees from Sanofi Genzyme. Claus Klingenberg received a consulting fee from Chiesi for being a member of the Nordic Neonatal Meeting Board and received no fees but has participated in the IMNUT study in Oslo, Norway, and in the Sensyn study in the United Kingdom.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

