Manipulating adrenergic stress receptor signalling to enhance immunosuppression and prolong survival of vascularized composite tissue transplants
- PMID: 35994413
- PMCID: PMC9394753
- DOI: 10.1002/ctm2.996
Manipulating adrenergic stress receptor signalling to enhance immunosuppression and prolong survival of vascularized composite tissue transplants
Abstract
Background: Vascularized composite tissue allotransplantation (VCA) to replace limbs or faces damaged beyond repair is now possible. The resulting clear benefit to quality of life is a compelling reason to attempt this complex procedure. Unfortunately, the high doses of immunosuppressive drugs required to protect this type of allograft result in significant morbidity and mortality giving rise to ethical concerns about performing this surgery in patients with non-life-threatening conditions. Here we tested whether we could suppress anti-graft immune activity by using a safe β2 -adrenergic receptor (AR) agonist, terbutaline, to mimic the natural immune suppression generated by nervous system-induced signalling through AR.
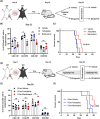
Methods: A heterotopic hind limb transplantation model was used with C57BL/6 (H-2b) as recipients and BALB/c (H-2d) mice as donors. To test the modulation of the immune response, graft survival was investigated after daily intraperitoneal injection of β2 -AR agonist with and without tacrolimus. Analyses of immune compositions and quantification of pro-inflammatory cytokines were performed to gauge functional immunomodulation. The contributions to allograft survival of β2 -AR signalling in donor and recipient tissue were investigated with β2 -AR-/- strains.
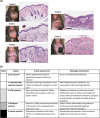
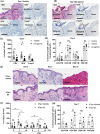
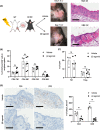
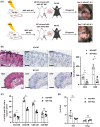
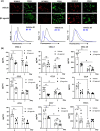
Results: Treatment with the β2 -AR agonist delayed VCA rejection, even with a subtherapeutic dose of tacrolimus. β2 -AR agonist decreased T-cell infiltration into the transplanted grafts and decreased memory T-cell populations in recipient's circulation. In addition, decreased levels of inflammatory cytokines (IFN-γ, IL-6, TNF-α, CXCL-1/10 and CCL3/4/5/7) were detected following β2 -AR agonist treatment, and there was a decreased expression of ICAM-1 and vascular cell adhesion molecule-1 in donor stromal cells.
Conclusions: β2 -AR agonist can be used safely to mimic the natural suppression of immune responses, which occurs during adrenergic stress-signalling and thereby can be used in combination regimens to reduce the dose needed of toxic immunosuppressive drugs such as tacrolimus. This strategy can be further evaluated for feasibility in the clinic.
Keywords: immunosuppression; stress signalling; vascularized composite tissue allotransplantation; β2-adrenergic receptors.
© 2022 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in the article.
Figures



Similar articles
-
Recipient bone marrow-derived stromal cells prolong graft survival in a rat hind limb allotransplantation model.Microsurgery. 2017 Sep;37(6):632-640. doi: 10.1002/micr.30128. Epub 2016 Nov 17. Microsurgery. 2017. PMID: 27859595
-
Intra-graft injection of tacrolimus promotes survival of vascularized composite allotransplantation.J Surg Res. 2017 Oct;218:49-57. doi: 10.1016/j.jss.2017.05.046. Epub 2017 Jun 10. J Surg Res. 2017. PMID: 28985877
-
The Effects of Tacrolimus on Tissue-Specific, Protein-Level Inflammatory Networks in Vascularized Composite Allotransplantation.Front Immunol. 2021 May 4;12:591154. doi: 10.3389/fimmu.2021.591154. eCollection 2021. Front Immunol. 2021. PMID: 34017323 Free PMC article.
-
Novel Strategies in Transplantation: Genetic Engineering and Vascularized Composite Allotransplantation.J Surg Res. 2023 Nov;291:176-186. doi: 10.1016/j.jss.2023.04.028. Epub 2023 Jul 8. J Surg Res. 2023. PMID: 37429217 Review.
-
Local immunosuppression in vascularized composite allotransplantation (VCA): A systematic review.J Plast Reconstr Aesthet Surg. 2021 Feb;74(2):327-335. doi: 10.1016/j.bjps.2020.10.003. Epub 2020 Oct 21. J Plast Reconstr Aesthet Surg. 2021. PMID: 33229219
Cited by
-
Chronic rejection models for vascularized composite tissue allotransplantation.Sci Rep. 2025 May 15;15(1):16882. doi: 10.1038/s41598-025-01803-8. Sci Rep. 2025. PMID: 40374749 Free PMC article.
References
-
- Fleming ME, Bharmal H, Valerio I. Regenerative medicine applications in combat casualty care. Regen Med. 2014;9(2):179‐190. - PubMed
-
- Fleming M, Waterman S, Dunne J, et al. Dismounted complex blast injuries: patterns of injuries and resource utilization associated with the multiple extremity amputee. J Surg Orthop Adv. 2012;21(1):32‐37. - PubMed
-
- Gawande A. Casualties of war–military care for the wounded from Iraq and Afghanistan. N Engl J Med. 2004;351(24):2471‐2475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
