The Influence of Sex, Body Mass Index, and Age on Cellular and Humoral Immune Responses Against Measles After a Third Dose of Measles-Mumps-Rubella Vaccine
- PMID: 35994504
- PMCID: PMC10205623
- DOI: 10.1093/infdis/jiac351
The Influence of Sex, Body Mass Index, and Age on Cellular and Humoral Immune Responses Against Measles After a Third Dose of Measles-Mumps-Rubella Vaccine
Abstract
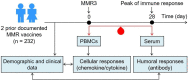
Background: A third dose of measles-mumps-rubella vaccine (MMR3) is recommended in mumps outbreak scenarios, but the immune response and the need for widespread use of MMR3 remain uncertain. Herein, we characterized measles-specific immune responses to MMR3 in a cohort of 232 healthy subjects.
Methods: Serum and peripheral blood mononuclear cells (PBMCs) were sampled at day 0 and day 28 after MMR3. Measles-specific binding and neutralizing antibodies were quantified in sera by enzyme-linked immunosorbent assay and a microneutralization assay, respectively. PBMCs were stimulated with inactivated measles virus, and the release of cytokines/chemokines was assessed by a multiplex assay. Demographic variables of subjects were examined for potential correlations with immune outcomes.
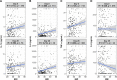
Results: Of the study participants, 95.69% and 100% were seropositive at day 0 and day 28, respectively. Antibody avidity significantly increased from 38.08% at day 0 to 42.8% at day 28 (P = .00026). Neutralizing antibodies were significantly enhanced, from 928.7 at day 0 to 1289.64 mIU/mL at day 28 (P = .0001). Meanwhile, cytokine/chemokine responses remained largely unchanged. Body mass index was significantly correlated with the levels of inflammatory cytokines/chemokines.
Conclusions: Measles-specific humoral immune responses, but not cellular responses, were enhanced after MMR3 receipt, extending current understanding of immune responses to MMR3 and supporting MMR3 administration to seronegative or high-risk individuals.
Keywords: MMR vaccine; MMR3; cellular immunity; humoral immunity; measles virus.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. G. A. P. offers consultative advice to Johnson & Johnson/Janssen Global Services LLC; is the chair of a safety evaluation committee for novel investigational vaccine trials being conducted by Merck Research Laboratories; and offers consultative advice on vaccine development to Merck & Co, Medicago, GlaxoSmithKline, Sanofi Pasteur, Emergent Biosolutions, Dynavax, Genentech, Eli Lilly, Kentucky Bioprocessing, Bavarian Nordic, AstraZeneca, Exelixis, Regeneron, Janssen, Vyriad, Moderna, and Genevant Sciences. G. A. P. and I. G. O. hold patents related to vaccinia and measles peptide vaccines. R. B. K., G. A. P., and I. G. O. hold a patent related to vaccinia peptide vaccines, and have received grant funding from ICW Ventures for preclinical studies on a peptide-based COVID-19 vaccine. G. A. P., R. B. K., I. G. O., and I. H. H. hold a patent related to the impact of single-nucleotide polymorphisms on measles vaccine immunity. R. B. K. has received funding from Merck Research Laboratories to study waning immunity to mumps vaccine, and also offers consultative advice on vaccine development to Merck & Co and Sanofi Pasteur. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

Similar articles
-
Measles Virus Neutralizing Antibody Response, Cell-Mediated Immunity, and Immunoglobulin G Antibody Avidity Before and After Receipt of a Third Dose of Measles, Mumps, and Rubella Vaccine in Young Adults.J Infect Dis. 2016 Apr 1;213(7):1115-23. doi: 10.1093/infdis/jiv555. Epub 2015 Nov 23. J Infect Dis. 2016. PMID: 26597262 Free PMC article.
-
Rubella virus-specific humoral immune responses and their interrelationships before and after a third dose of measles-mumps-rubella vaccine in women of childbearing age.Vaccine. 2020 Jan 29;38(5):1249-1257. doi: 10.1016/j.vaccine.2019.11.004. Epub 2019 Nov 12. Vaccine. 2020. PMID: 31732325 Free PMC article.
-
Adverse Events Among Young Adults Following a Third Dose of Measles-Mumps-Rubella Vaccine.Clin Infect Dis. 2021 Oct 5;73(7):e1546-e1553. doi: 10.1093/cid/ciaa1090. Clin Infect Dis. 2021. PMID: 32766827
-
Vaccines for measles, mumps, rubella, and varicella in children.Cochrane Database Syst Rev. 2020 Apr 20;4(4):CD004407. doi: 10.1002/14651858.CD004407.pub4. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Nov 22;11:CD004407. doi: 10.1002/14651858.CD004407.pub5. PMID: 32309885 Free PMC article. Updated.
-
Two doses of MMR vaccine--sufficient to eradicate measles, mumps and rubella?Scand J Soc Med. 1988;16(3):129-35. doi: 10.1177/140349488801600301. Scand J Soc Med. 1988. PMID: 3057621 Review.
Cited by
-
Early B cell transcriptomic markers of measles-specific humoral immunity following a 3rd dose of MMR vaccine.Front Immunol. 2024 Apr 3;15:1358477. doi: 10.3389/fimmu.2024.1358477. eCollection 2024. Front Immunol. 2024. PMID: 38633249 Free PMC article.
-
Virus-specific and shared gene expression signatures in immune cells after vaccination in response to influenza and vaccinia stimulation.Front Immunol. 2023 Aug 4;14:1168784. doi: 10.3389/fimmu.2023.1168784. eCollection 2023. Front Immunol. 2023. PMID: 37600811 Free PMC article.
-
Seronegative Vaccinees May Not Benefit From Multiple Booster Doses of MMR Vaccine in Restoring Immunity.J Med Virol. 2024 Dec;96(12):e70135. doi: 10.1002/jmv.70135. J Med Virol. 2024. PMID: 39722553 Free PMC article.
-
Seroprevalence of Varicella-Zoster Virus and Measles among Healthcare Workers in a Tertiary Medical Center in Korea.Vaccines (Basel). 2022 Nov 18;10(11):1956. doi: 10.3390/vaccines10111956. Vaccines (Basel). 2022. PMID: 36423051 Free PMC article.
-
T Cell Transcriptional Signatures of Influenza A/H3N2 Antibody Response to High Dose Influenza and Adjuvanted Influenza Vaccine in Older Adults.Viruses. 2022 Dec 11;14(12):2763. doi: 10.3390/v14122763. Viruses. 2022. PMID: 36560767 Free PMC article. Clinical Trial.
References
-
- Cheng VC, Wong SC, Wong SC, et al. Measles outbreak from Hong Kong International Airport to the hospital due to secondary vaccine failure in healthcare workers. Infect Control Hosp Epidemiol 2019; 40:1407–15. - PubMed
-
- Hahné SJ, Nic Lochlainn LM, van Burgel ND, et al. Measles outbreak among previously immunized healthcare workers, the Netherlands, 2014. J Infect Dis 2016; 214:1980–6. - PubMed
-
- Rosen JB, Rota JS, Hickman CJ, et al. Outbreak of measles among persons with prior evidence of immunity, New York City, 2011. Clin Infect Dis 2014; 58:1205–10. - PubMed
-
- Song K, Lee JM, Lee EJ, et al. Control of a nosocomial measles outbreak among previously vaccinated adults in a population with high vaccine coverage: Korea, 2019. Eur J Clin Microbiol Infect Dis 2022; 41:455–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

