The performance of using dried blood spot specimens for HIV-1 viral load testing: A systematic review and meta-analysis
- PMID: 35994520
- PMCID: PMC9447868
- DOI: 10.1371/journal.pmed.1004076
The performance of using dried blood spot specimens for HIV-1 viral load testing: A systematic review and meta-analysis
Abstract
Background: Accurate routine HIV viral load testing is essential for assessing the efficacy of antiretroviral treatment (ART) regimens and the emergence of drug resistance. While the use of plasma specimens is the standard for viral load testing, its use is restricted by the limited ambient temperature stability of viral load biomarkers in whole blood and plasma during storage and transportation and the limited cold chain available between many health care facilities in resource-limited settings. Alternative specimen types and technologies, such as dried blood spots, may address these issues and increase access to viral load testing; however, their technical performance is unclear. To address this, we conducted a meta-analysis comparing viral load results from paired dried blood spot and plasma specimens analyzed with commonly used viral load testing technologies.
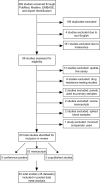
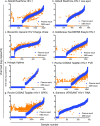
Methods and findings: Standard databases, conferences, and gray literature were searched in 2013 and 2018. Nearly all studies identified (60) were conducted between 2007 and 2018. Data from 40 of the 60 studies were included in the meta-analysis, which accounted for a total of 10,871 paired dried blood spot:plasma data points. We used random effects models to determine the bias, accuracy, precision, and misclassification for each viral load technology and to account for between-study variation. Dried blood spot specimens produced consistently higher mean viral loads across all technologies when compared to plasma specimens. However, when used to identify treatment failure, each technology compared best to plasma at a threshold of 1,000 copies/ml, the present World Health Organization recommended treatment failure threshold. Some heterogeneity existed between technologies; however, 5 technologies had a sensitivity greater than 95%. Furthermore, 5 technologies had a specificity greater than 85% yet 2 technologies had a specificity less than 60% using a treatment failure threshold of 1,000 copies/ml. The study's main limitation was the direct applicability of findings as nearly all studies to date used dried blood spot samples prepared in laboratories using precision pipetting that resulted in consistent input volumes.
Conclusions: This analysis provides evidence to support the implementation and scale-up of dried blood spot specimens for viral load testing using the same 1,000 copies/ml treatment failure threshold as used with plasma specimens. This may support improved access to viral load testing in resource-limited settings lacking the required infrastructure and cold chain storage for testing with plasma specimens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- UNAIDS. Ending AIDS: Progress towards the 90-90-90 targets. 2017.
-
- Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, et al.. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. - PubMed
-
- Rodger A, Cambiano V, Bruun T, Vernazza P, Collins S, Degen O, et al.. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet. 2019;393(10189):2428–2438. doi: 10.1016/S0140-6736(19)30418-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

