Isolation of a virus causing a chronic infection in the archaeal model organism Haloferax volcanii reveals antiviral activities of a provirus
- PMID: 35994644
- PMCID: PMC9436352
- DOI: 10.1073/pnas.2205037119
Isolation of a virus causing a chronic infection in the archaeal model organism Haloferax volcanii reveals antiviral activities of a provirus
Abstract
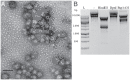
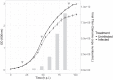
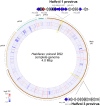
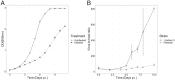
Viruses are important ecological, biogeochemical, and evolutionary drivers in every environment. Upon infection, they often cause the lysis of the host cell. However, some viruses exhibit alternative life cycles, such as chronic infections without cell lysis. The nature and the impact of chronic infections in prokaryotic host organisms remains largely unknown. Here, we characterize a novel haloarchaeal virus, Haloferax volcanii pleomorphic virus 1 (HFPV-1), which is currently the only virus infecting the model haloarchaeon Haloferax volcanii DS2, and demonstrate that HFPV-1 and H. volcanii are a great model system to study virus-host interactions in archaea. HFPV-1 is a pleomorphic virus that causes a chronic infection with continuous release of virus particles, but host and virus coexist without cell lysis or the appearance of resistant cells. Despite an only minor impact of the infection on host growth, we uncovered an extensive remodeling of the transcriptional program of the host (up to 1,049 differentially expressed genes). These changes are highlighted by a down-regulation of two endogenous provirus regions in the host genome, and we show that HFPV-1 infection is strongly influenced by a cross-talk between HFPV-1 and one of the proviruses mediated by a superinfection-like exclusion mechanism. Furthermore, HFPV-1 has a surprisingly wide host range among haloarchaea, and purified virus DNA can cause an infection after transformation into the host, making HFPV-1 a candidate for being developed into a genetic tool for a range of so far inaccessible haloarchaea.
Keywords: CRISPR; archaea; chronic infection; virus.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Cobián Güemes A. G., et al. , Viruses as winners in the game of life. Annu. Rev. Virol. 3, 197–214 (2016). - PubMed
-
- Wilhelm S. W., Suttle C. A., Viruses and nutrient cycles in the sea aquatic food webs. Bioscience 49, 781–788 (1999).
-
- Zimmerman A. E., et al. , Metabolic and biogeochemical consequences of viral infection in aquatic ecosystems. Nat. Rev. Microbiol. 18, 21–34 (2019). - PubMed
-
- Maniloff J., Das J., Christensen J. R., Viruses of mycoplasmas and spiroplasmas. Adv. Virus Res. 21, 343–380 (1977). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

