Malaria parasite evades mosquito immunity by glutaminyl cyclase-mediated posttranslational protein modification
- PMID: 35994647
- PMCID: PMC9436314
- DOI: 10.1073/pnas.2209729119
Malaria parasite evades mosquito immunity by glutaminyl cyclase-mediated posttranslational protein modification
Abstract
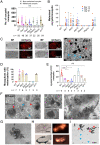
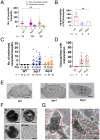
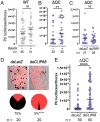
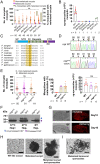
Glutaminyl cyclase (QC) modifies N-terminal glutamine or glutamic acid residues of target proteins into cyclic pyroglutamic acid (pGlu). Here, we report the biochemical and functional analysis of Plasmodium QC. We show that sporozoites of QC-null mutants of rodent and human malaria parasites are recognized by the mosquito immune system and melanized when they reach the hemocoel. Detailed analyses of rodent malaria QC-null mutants showed that sporozoite numbers in salivary glands are reduced in mosquitoes infected with QC-null or QC catalytically dead mutants. This phenotype can be rescued by genetic complementation or by disrupting mosquito melanization or phagocytosis by hemocytes. Mutation of a single QC-target glutamine of the major sporozoite surface protein (circumsporozoite protein; CSP) of the rodent parasite Plasmodium berghei also results in melanization of sporozoites. These findings indicate that QC-mediated posttranslational modification of surface proteins underlies evasion of killing of sporozoites by the mosquito immune system.
Keywords: glutaminyl cyclase; immune evasion; melanization; pyroglutamic acid; sporozoite.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Wintjens R., et al. , Crystal structure of papaya glutaminyl cyclase, an archetype for plant and bacterial glutaminyl cyclases. J. Mol. Biol. 357, 457–470 (2006). - PubMed
-
- Schilling S., Wasternack C., Demuth H. U., Glutaminyl cyclases from animals and plants: A case of functionally convergent protein evolution. Biol. Chem. 389, 983–991 (2008). - PubMed
-
- Vijayan D. K., Zhang K. Y. J., Human glutaminyl cyclase: Structure, function, inhibitors and involvement in Alzheimer’s disease. Pharmacol. Res. 147, 104342 (2019). - PubMed
-
- Saido T. C., et al. , Dominant and differential deposition of distinct beta-amyloid peptide species, A beta N3(pE), in senile plaques. Neuron 14, 457–466 (1995). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

