Glycemic Variability Patterns Strongly Correlate With Partial Remission Status in Children With Newly Diagnosed Type 1 Diabetes
- PMID: 35994729
- PMCID: PMC9862313
- DOI: 10.2337/dc21-2543
Glycemic Variability Patterns Strongly Correlate With Partial Remission Status in Children With Newly Diagnosed Type 1 Diabetes
Abstract
Objective: To evaluate whether indexes of glycemic variability may overcome residual β-cell secretion estimates in the longitudinal evaluation of partial remission in a cohort of pediatric patients with new-onset type 1 diabetes.
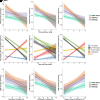
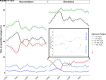
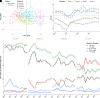
Research design and methods: Values of residual β-cell secretion estimates, clinical parameters (e.g., HbA1c or insulin daily dose), and continuous glucose monitoring (CGM) from 78 pediatric patients with new-onset type 1 diabetes were longitudinally collected during 1 year and cross-sectionally compared. Circadian patterns of CGM metrics were characterized and correlated to remission status using an adjusted mixed-effects model. Patients were clustered based on 46 CGM metrics and clinical parameters and compared using nonparametric ANOVA.
Results: Study participants had a mean (± SD) age of 10.4 (± 3.6) years at diabetes onset, and 65% underwent partial remission at 3 months. β-Cell residual secretion estimates demonstrated weak-to-moderate correlations with clinical parameters and CGM metrics (r2 = 0.05-0.25; P < 0.05). However, CGM metrics strongly correlated with clinical parameters (r2 >0.52; P < 0.05) and were sufficient to distinguish remitters from nonremitters. Also, CGM metrics from remitters displayed specific early morning circadian patterns characterized by increased glycemic stability across days (within 63-140 mg/dL range) and decreased rate of grade II hypoglycemia (P < 0.0001) compared with nonremitters. Thorough CGM analysis allowed the identification of four novel glucotypes (P < 0.001) that segregate patients into subgroups and mirror the evolution of remission after diabetes onset.
Conclusions: In our pediatric cohort, combination of CGM metrics and clinical parameters unraveled key clinical milestones of glucose homeostasis and remission status during the first year of type 1 diabetes.
Trial registration: ClinicalTrials.gov NCT04007809.
© 2022 by the American Diabetes Association.
Figures
References
-
- van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 2011;91:79–118 - PubMed
-
- Palmer JP, Fleming GA, Greenbaum CJ, et al. . C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001. Diabetes 2004;53:250–264 - PubMed

