Ultramicroporous material based parallel and extended paraffin nano-trap for benchmark olefin purification
- PMID: 35995798
- PMCID: PMC9395351
- DOI: 10.1038/s41467-022-32677-3
Ultramicroporous material based parallel and extended paraffin nano-trap for benchmark olefin purification
Abstract
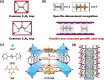
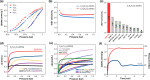
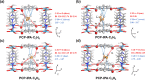
Selective paraffin capture from olefin/paraffin mixtures could afford high-purity olefins directly, but suffers from the issues of low separation selectivity and olefin productivity. Herein, we report an ultramicroporous material (PCP-IPA) with parallel-aligned linearly extending isophthalic acid units along the one-dimensional channel, realizing the efficient production of ultra-high purity C2H4 and C3H6 (99.99%). The periodically expanded and parallel-aligned aromatic-based units served as a paraffin nano-trap to contact with the exposed hydrogen atoms of both C2H6 and C3H8, as demonstrated by the simulation studies. PCP-IPA exhibits record separation selectivity of 2.48 and separation potential of 1.20 mol/L for C3H8/C3H6 (50/50) mixture, meanwhile the excellent C2H6/C2H4 mixture separation performance. Ultra-high purity C3H6 (99.99%) and C2H4 (99.99%) can be directly obtained through fixed-bed column from C3H8/C3H6 and C2H6/C2H4 mixtures, respectively. The record C3H6 productivity is up to 15.23 L/kg from the equimolar of C3H8/C3H6, which is 3.85 times of the previous benchmark material, demonstrating its great potential for those important industrial separations.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Fine-Tuning and Selective-Binding within an Anion-Functionalized Ultramicroporous Metal-Organic Framework for Efficient Olefin/Paraffin Separation.ACS Appl Mater Interfaces. 2020 Sep 9;12(36):40229-40235. doi: 10.1021/acsami.0c07800. Epub 2020 Aug 28. ACS Appl Mater Interfaces. 2020. PMID: 32805845
-
Ultramicroporous Hydrogen-Bonded Organic Framework Material with a Thermoregulatory Gating Effect for Record Propylene Separation.J Am Chem Soc. 2022 Sep 21;144(37):17033-17040. doi: 10.1021/jacs.2c06585. Epub 2022 Sep 7. J Am Chem Soc. 2022. PMID: 36069372
-
Efficient One-Step Purification of Methanol-to-Olefin Products Using a Porphyrinyl MOF to Achieve Record C2H4 and C3H6 Productivity.ACS Appl Mater Interfaces. 2025 Apr 9;17(14):21630-21642. doi: 10.1021/acsami.4c21500. Epub 2025 Mar 29. ACS Appl Mater Interfaces. 2025. PMID: 40156512
-
Pore-Space Partition and Optimization for Propane-Selective High-Performance Propane/Propylene Separation.ACS Appl Mater Interfaces. 2021 Nov 10;13(44):52160-52166. doi: 10.1021/acsami.1c10391. Epub 2021 Jul 8. ACS Appl Mater Interfaces. 2021. PMID: 34236170 Review.
-
Alternatives to Cryogenic Distillation: Advanced Porous Materials in Adsorptive Light Olefin/Paraffin Separations.Small. 2019 Jun;15(25):e1900058. doi: 10.1002/smll.201900058. Epub 2019 Apr 17. Small. 2019. PMID: 30993886 Review.
Cited by
-
Probing sub-5 Ångstrom micropores in carbon for precise light olefin/paraffin separation.Nat Commun. 2023 Mar 2;14(1):1197. doi: 10.1038/s41467-023-36890-6. Nat Commun. 2023. PMID: 36864084 Free PMC article.
-
Oriented design and engineering of advanced metal-organic frameworks for light hydrocarbon separations.Chem Sci. 2025 Jun 2;16(26):11768-11800. doi: 10.1039/d5sc01755f. eCollection 2025 Jul 2. Chem Sci. 2025. PMID: 40538895 Free PMC article. Review.
-
Pillar-layer Zn-triazolate-dicarboxylate frameworks with a customized pore structure for efficient ethylene purification from ethylene/ethane/acetylene ternary mixtures.Chem Sci. 2023 May 15;14(22):5912-5917. doi: 10.1039/d3sc01134h. eCollection 2023 Jun 7. Chem Sci. 2023. PMID: 37293648 Free PMC article.
-
Recent Advances of Multidentate Ligand-Based Anion-Pillared MOFs for Enhanced Separation and Purification Processes.Chem Bio Eng. 2024 Apr 10;1(6):469-487. doi: 10.1021/cbe.3c00115. eCollection 2024 Jul 25. Chem Bio Eng. 2024. PMID: 39974605 Free PMC article. Review.
-
Efficient Selective Capture of Carbon Dioxide from Nitrogen and Methane Using a Metal-Organic Framework-Based Nanotrap.Molecules. 2023 Dec 2;28(23):7908. doi: 10.3390/molecules28237908. Molecules. 2023. PMID: 38067637 Free PMC article.
References
Grants and funding
- 22108240/National Natural Science Foundation of China (National Science Foundation of China)
- 21938011/National Natural Science Foundation of China (National Science Foundation of China)
- 21725603/National Natural Science Foundation of China (National Science Foundation of China)
- LR20B060001/Natural Science Foundation of Zhejiang Province (Zhejiang Provincial Natural Science Foundation)
LinkOut - more resources
Full Text Sources
Other Literature Sources

