SOCS2-enhanced ubiquitination of SLC7A11 promotes ferroptosis and radiosensitization in hepatocellular carcinoma
- PMID: 35995846
- PMCID: PMC9883449
- DOI: 10.1038/s41418-022-01051-7
SOCS2-enhanced ubiquitination of SLC7A11 promotes ferroptosis and radiosensitization in hepatocellular carcinoma
Abstract
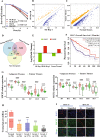
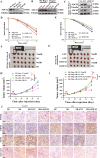
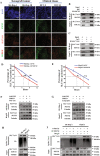
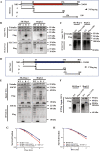
Radioresistance is a principal culprit for the failure of radiotherapy in hepatocellular carcinoma (HCC). Insights on the regulation genes of radioresistance and underlying mechanisms in HCC are awaiting for profound investigation. In this study, the suppressor of cytokine signaling 2 (SOCS2) were screened out by RNA-seq and bioinformatics analyses as a potential prognosis predictor of HCC radiotherapy and then were determined to promote radiosensitivity in HCC both in vivo or in vitro. Meanwhile, the measurements of ferroptosis negative regulatory proteins of solute carrier family 7 member 11 (SLC7A11) and glutathione peroxidase 4 (GPX4), intracellular lipid peroxidation and Fe2+ concentration suggested that a high level of ferroptosis contributed to the radiosensitization of HCC. Moreover, SOCS2 and SLC7A11 were expressed oppositely in HCC clinical tissues and tumour xenografts with different radiosensitivities. Mechanistically, the N-terminal domain of SLC7A11 was specifically recognized by the SH2-structural domain of SOCS2. While the L162 and C166 of SOCS2-BOX region could bind elongin B/C compound to co-form a SOCS2/elongin B/C complex to recruit ubiquitin molecules. Herein, SOCS2 served as a bridge to transfer the attached ubiquitin to SLC7A11 and promoted K48-linked polyubiquitination degradation of SLC7A11, which ultimately led to the onset of ferroptosis and radiosensitization of HCC. In conclusion, it was demonstrated for the first time that high-expressed SOCS2 was one of the biomarkers predicting radiosensitivity of HCC by advancing the ubiquitination degradation of SLC7A11 and promoting ferroptosis, which indicates that targeting SOCS2 may enhance the efficiency of HCC radiotherapy and improve the prognosis of patients.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

