Natural immunity to SARS-CoV-2 and breakthrough infections in vaccinated and unvaccinated patients with cancer
- PMID: 35995934
- PMCID: PMC9395853
- DOI: 10.1038/s41416-022-01952-x
Natural immunity to SARS-CoV-2 and breakthrough infections in vaccinated and unvaccinated patients with cancer
Abstract
Background: Consolidated evidence suggests spontaneous immunity from SARS-CoV-2 is not durable, leading to the risk of reinfection, especially in the context of newly emerging viral strains. In patients with cancer who survive COVID-19 prevalence and severity of SARS-CoV-2 reinfections are unknown.
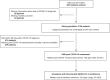
Methods: We aimed to document natural history and outcome from SARS-CoV-2 reinfection in patients recruited to OnCovid (NCT04393974), an active European registry enrolling consecutive patients with a history of solid or haematologic malignancy diagnosed with COVID-19.
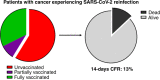
Results: As of December 2021, out of 3108 eligible participants, 1806 COVID-19 survivors were subsequently followed at participating institutions. Among them, 34 reinfections (1.9%) were reported after a median time of 152 days (range: 40-620) from the first COVID-19 diagnosis, and with a median observation period from the second infection of 115 days (95% CI: 27-196). Most of the first infections were diagnosed in 2020 (27, 79.4%), while most of reinfections in 2021 (25, 73.5%). Haematological malignancies were the most frequent primary tumour (12, 35%). Compared to first infections, second infections had lower prevalence of COVID-19 symptoms (52.9% vs 91.2%, P = 0.0008) and required less COVID-19-specific therapy (11.8% vs 50%, P = 0.0013). Overall, 11 patients (32.4%) and 3 (8.8%) were fully and partially vaccinated against SARS-CoV-2 before the second infection, respectively. The 14-day case fatality rate was 11.8%, with four death events, none of which among fully vaccinated patients.
Conclusion: This study shows that reinfections in COVID-19 survivors with cancer are possible and more common in patients with haematological malignancies. Reinfections carry a 11% risk of mortality, which rises to 15% among unvaccinated patients, highlighting the importance of universal vaccination of patients with cancer.
© 2022. The Author(s).
Conflict of interest statement
AC received consulting fees from MSD, BMS, AstraZeneca, Roche; speakers’ fee from AstraZeneca, MSD, Novartis and Eisai. AG has declared consulting/advisory role for Roche, MSD, Eli Lilly, Pierre Fabre, EISAI and Daichii Sankyo; speakers bureau for Eisai, Novartis, Eli Lilly, Roche, Teva, Gentili, Pfizer, AstraZeneca, Celgene and Daichii Sankyo; research funds: EISAI, Eli Lilly, and Roche. DJP received lecture fees from ViiV Healthcare, Bayer Healthcare, BMS, Roche, EISAI, Falk Foundation, travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, EISAI, Roche, DaVolterra and AstraZeneca; research funding (to institution) from MSD and BMS. The remaining authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

