Atezolizumab versus chemotherapy in advanced or metastatic NSCLC with high blood-based tumor mutational burden: primary analysis of BFAST cohort C randomized phase 3 trial
- PMID: 35995953
- PMCID: PMC9499854
- DOI: 10.1038/s41591-022-01933-w
Atezolizumab versus chemotherapy in advanced or metastatic NSCLC with high blood-based tumor mutational burden: primary analysis of BFAST cohort C randomized phase 3 trial
Abstract
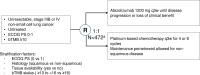
Tumor mutational burden (TMB) is being explored as a predictive biomarker for cancer immunotherapy outcomes in non-small cell lung cancer. BFAST (NCT03178552)-an open-label, global, multicohort trial-evaluated the safety and efficacy of first-line targeted therapies or immunotherapy in patients with unresectable Stage IIIB or IV advanced or metastatic non-small cell lung cancer who were selected for biomarker status using blood-based targeted next-generation sequencing. In the Phase 3 cohort C evaluating blood-based (b)TMB as a biomarker of atezolizumab efficacy, patients with bTMB of ≥10 (N = 471) were randomized 1:1 to receive atezolizumab or platinum-based chemotherapy per local standard of care. Cohort C did not meet its primary endpoint of investigator-assessed progression-free survival in the population with bTMB of ≥16 (hazard ratio, 0.77; 95% confidence interval: 0.59, 1.00; P = 0.053). Adverse events leading to treatment withdrawal occurred in 10% of patients in the atezolizumab arm and 20% in the chemotherapy arm. Adverse events of special interest occurred in 42% of patients in the atezolizumab arm and 26% in the chemotherapy arm. A prespecified exploratory analysis compared the bTMB clinical trial assay with the FoundationOne Liquid Companion Diagnostic assay and showed high concordance between assays. Additional exploration of bTMB to identify optimal cutoffs, confounding factors, assay improvements or cooperative biomarkers is warranted.
© 2022. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: S.P. has received institutional support for consulting or advising from AbbVie, Amgen, AstraZeneca, Bayer, BeiGene, Biocartis, Boehringer Ingelheim, Bristol Myers Squibb, Clovis, Daiichi Sankyo, Debiopharm, eCancer, Eli Lilly, Elsevier, Foundation Medicine, Illumina, Imedex, IQVIA, Incyte, Janssen, Medscape, Merck Sharp & Dohme, Merck Serono, Merrimack, Novartis, Oncology Education, PharmaMar, Phosplatin Therapeutics, PER, Pfizer, PRIME, Regeneron, RMEI, Roche/Genentech, RTP, Sanofi, Seattle Genetics and Takeda; institutional fees for speaking at company-sponsored public events for AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, eCancer, Eli Lilly, Illumina, Imedex, Medscape, Merck Sharp & Dohme, Novartis, PER, Pfizer, Prime, Roche/Genentech, RTP, Sanofi and Takeda; and received institutional grants and research support for the conduct of clinical trials from Amgen, AstraZeneca, Biodesix, Boehringer Ingelheim, Bristol Myers Squibb, Clovis, GlaxoSmithKline, Illumina, Lilly, Merck Sharp & Dohme, Merck Serono, Mirati, Novartis, Pfizer, Phosplatin Therapeutics and Roche/Genentech. R.D. has received fees for advisory boards or speaking from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, F. Hoffmann-La Roche, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Pfizer, Regeneron, Seattle Genetics, Takeda and Karyopharm. A.M. has received fees for advisory boards or speaking from Bristol Myers Squibb, Merck Sharpe & Dohme, Roche, AstraZeneca, Pfizer, Novartis, Novartis, Takeda, and Boehringer Ingelheim. E.F. has received fees for advisory boards or speaking from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, F. Hoffmann-La Roche, GlaxoSmithKline, Janssen, Medscape, Merck KGaA, Merck Sharp & Dohme, Novartis, Peptomyc, PeerVoice, Pfizer, Regeneron, Sanofi Genzyme, Syneos Health, Seattle Genetics, Takeda and Touch Medical; served as an independent board member for Grifols; and received research funding from Fundaciόn Merck Salud, Grant for Oncology Innovation and Merck Health. S.M.G. has received fees for consulting from Genentech/Roche, Takeda, AstraZeneca, Pfizer, Daiichi Sankyo and Eli Lilly, and has served on an independent data monitoring committee for AstraZeneca. P.C. has received fees for serving on advisory boards for AstraZeneca, Bristol Myers Squibb, BeiGene, Amgen, Novartis, Pfizer, EMD Serono, Bayer, Janssen, Merck, and F. Hoffmann-La Roche. C.H.B. has received institutional grants and research support from Pfizer, Novartis, Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Roche/Genentech, Lilly, Sanofi, Taiho Pharmaceutical, Mylan, Merrimack, Merck, AbbVie, Astellas Pharma, BioMarin, Bristol Myers Squibb, Daiichi Sankyo, Abraxis Biosciences, AB Science, Asana Biosciences, Medivation, Exelixis, ImClone Systems, LEO Pharma, Millenium, Merck KGaA, Shanghai Henlius Biotech, Polyphor and PharmaMar; has ownership or holds stock in Tummi and MEDSir; and has received fees for consulting or serving on advisory boards from Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Roche/Genentech, Eisai, Bayer, Merck Sharp & Dohme, AstraZeneca, Zodiac, Lilly and Sanofi. P.D. has received fees for consulting or serving on advisory boards from Merck Sharpe & Dohme, Roche, and Bristol Myers Squibb; and institutional grants from Merck Sharpe & Dohme, Roche, and AstraZeneca. M.J. has received institutional funding research from AbbVie, Acerta, Adaptimmune, Amgen, Apexigen, Arcus Biosciences, Array BioPharma, Artios Pharma, AstraZeneca, Atreca, BeiGene, BerGenBio, BioAtla, Boehringer Ingelheim, Calithera Biosciences, Checkpoint Therapeutics, Corvus Pharmaceuticals, Curis, CytomX, Daiichi Sankyo, Dracen Pharmaceuticals, Dynavax, Lilly, Elicio Therapeutics, EMD Serono, Erasca, Genentech/Roche, Genmab, Genocea Biosciences, GlaxoSmithKline, Gritstone Oncology, Guardant Health, Harpoon, Helsinn Healthcare SA, Hengrui Therapeutics, Hutchison MediPharma, IDEAYA Biosciences, IGM Biosciences, Immunocore, Incyte, Janssen, Jounce Therapeutics, Kadmon Pharmaceuticals, Loxo Oncology, Lycera, Memorial Sloan-Kettering, Merck, Mirati Therapeutics, NeoImmune Tech, Neovia Oncology, Novartis, Numab Therapeutics, OncoMed Pharmaceuticals, Pfizer, PMV Pharmaceuticals, RasCal Therapeutics, Regeneron Pharmaceuticals, Relay Therapeutics, Revolution Medicines, Ribon Therapeutics, Rubius Therapeutics, Sanofi, Seven and Eight Biopharmaceuticals/Birdie Biopharmaceuticals, Shattuck Labs, Silicon Therapeutics, Stem CentRx, Syndax Pharmaceuticals, Takeda Pharmaceuticals, Tarveda, TCR2 Therapeutics, Tempest Therapeutics, Tizona Therapeutics, TMUNITY Therapeutics, Turning Point Therapeutics, University of Michigan, Vyriad, WindMIL and Y-mAbs Therapeutics; and institutional funding for consulting and advisory roles from AbbVie, Achilles Therapeutics, Amgen, AstraZeneca, Axelia Oncology, Atreca, Black Diamond, Boehringer Ingelheim, Bristol Myers Squibb, Calithera Biosciences, Checkpoint Therapeutics, CytomX Therapeutics, Daiichi Sankyo, EcoR1, Editas Medicine, Eisai, EMD Serono, G1 Therapeutics, Genentech/Roche, Genmab, GlaxoSmithKline, Gritstone Oncology, Guardant Health, Ideaya Biosciences, iTeos, Incyte, Janssen, Lilly, Loxo Oncology, Merck, Mirati Therapeutics, Novartis, Oncorus, Pfizer, Regeneron Pharmaceuticals, Ribon Therapeutics, Sanofi-Aventis, Turning Point Therapeutics, and WindMIL. S.N. has served on speaker’s bureaus and as an advisor for AstraZeneca, Boehringer Ingelheim, BeiGene, Bayer, Roche/Genentech, Merck Sharp & Dohme, Pfizer, Takeda, Eli Lilly, AMG, Novartis, Sanofi and GlaxoSmithKline. D.R.G. has received institutional research grants from Amgen, AstraZeneca, Merck, and Roche/Genentech; personal fees for consulting or advisory roles for Inivata, Lilly, Merck, and Novartis; and institutional grants with no personal income for consulting or advisory roles for AstraZeneca, Roche/Genentech, Guardant Health, IO Biotech, and Oncocyte. M.S.M. and D.V. are employees of Genentech. S.M.S., J.W. and E.S. are employees and stockholders of Genentech. M. Yan is an employee of F. Hoffmann-La Roche Ltd. S.M. was an employee of and holds stock in Roche/Genentech. D.A.F. is an employee of Foundation Medicine and stockholder in Roche/Genentech. D.S.S. is an employee of Genentech and owns stock in Roche Holdings. T.R. was an employee of Genentech. T.M. has received fees for serving on advisory boards and consulting, and speakers fees and institutional grants and research support from Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Pfizer; has received fees for serving on advisory boards and consulting and speakers fees from ACEA Pharma, Amgen, Boehringer lngelheim Pharmaceuticals, Inc., Daiichi Sankyo, Inc., Fishawack Facilitate, Ltd., Lilly, OrigiMed Co. Ltd., Sanofi-Aventis; owns stock and has received fees for serving on advisory boards and board of directors/leadership roles from HutchMed; institutional grants and research support and fees for serving on advisory boards and consulting from Merck Serono and SFJ Pharmaceutical Ltd.; has received fees for serving on advisory boards, board of directors/leadership roles and consulting from Lunit, Inc. fees for serving on advisory boards and for consulting from AbbVie Inc., BerryOncology, Blueprint Medicines Corporation, C4 Therapeutics, Inc, CStone Pharmaceuticals, Curio Science, Eisai, Gilead Sciences, Inc., Gritstone Oncology,Inc., Guardant Health, Hengrui Therapeutics Inc., IQVIA, Janssen, lgnyta, Inc., lncyte Corporation, lnivata, Loxo Oncology Inc., Mirati Therapeutics Inc., Puma Biotechnology Inc., Vertex Pharmaceuticals, Yuhan Corporation; has received speakers fees and fees for consulting from Alpha Biopharma Co., Ltd., Amoy Diagnostics Co., Ltd., AstraZeneca (before 1 January 2019), BeiGene; has received fees for serving on advisory boards and institutional grants and research support from AstraZeneca, Gl Therapeutics, Inc., Takeda; institutional grants and research support from Roche, XCovery; has received speakers fees from Daz Group, InMed Medical Communication, Janssen Pharmaceutica NV, Liangyihui Network Technology Co., Ltd., Lucence Health Inc., MD Health Brazil, Medscape LLC, Merck Pharmaceuticals HK Ltd., P. Permanyer SL, PeerVoice, Physicians’ Education Resource, PrIME Oncology, Research to Practice, Roche Pharmaceuticals/Diagnostic/Foundation One, Shanghai BeBirds Translation and Consulting Co., Ltd., Taiho, Takeda Oncology, touchIME; has received fees for consulting from Elevation Oncology, MoreHealth, Qiming Development (HK) Ltd., Roche Pharmaceuticals, Takeda Pharmaceuticals HK Ltd.; has received fees for serving on advisory boards for Roche/Genentech and Virtus Medical Group; has received fees for a board of directors/leadership role with AstraZeneca PLC; discloses serving on advisory boards (uncompensated) for geneDecode Co.,Ltd.; owns stock from Act Genomics-Sanomics Group and Aurora Tele-Oncology Ltd.; declares uncompensated board of directors/leadership roles with the American Society of Clinical Oncology, Asian Thoracic Oncology Research Group, Chinese Lung Cancer Research Foundation Limited, Chinese Society of Clinical Oncology, Hong Kong Cancer Fund, Hong Kong Cancer Therapy Society, International Association for the Study of Lung Cancer (ending 30 April 2019), St. Stephen’s College & Preparatory School. M.C., M.Yamaguchi., E.D. and Z.A. have declared that they have no competing interests.
Figures







Comment in
-
Liquid biopsies and tumor mutational burden: the cutoff conundrum.Nat Med. 2022 Sep;28(9):1753-1754. doi: 10.1038/s41591-022-01999-6. Nat Med. 2022. PMID: 36097224 No abstract available.
-
BFAST but be smart: bTMB remains an exploratory biomarker in NSCLC.Nat Rev Clin Oncol. 2023 Jan;20(1):3-4. doi: 10.1038/s41571-022-00698-y. Nat Rev Clin Oncol. 2023. PMID: 36271141 No abstract available.
References
-
- Marabelle A, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–1365. - PubMed
-
- Keytruda (pembrolizumab). Prescribing Information (MSD International GmbH, 2020).
-
- Kowanetz M, et al. OA20.01 Tumor mutation burden (TMB) is associated with improved efficacy of atezolizumab in 1L and 2L+ NSCLC patients. J. Thorac. Oncol. 2017;12:S321–S322.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

