Artificial intelligence-enabled detection and assessment of Parkinson's disease using nocturnal breathing signals
- PMID: 35995955
- PMCID: PMC9556299
- DOI: 10.1038/s41591-022-01932-x
Artificial intelligence-enabled detection and assessment of Parkinson's disease using nocturnal breathing signals
Abstract
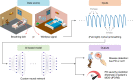
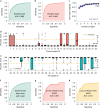
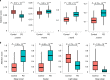
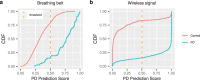
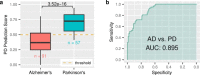
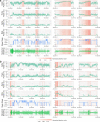
There are currently no effective biomarkers for diagnosing Parkinson's disease (PD) or tracking its progression. Here, we developed an artificial intelligence (AI) model to detect PD and track its progression from nocturnal breathing signals. The model was evaluated on a large dataset comprising 7,671 individuals, using data from several hospitals in the United States, as well as multiple public datasets. The AI model can detect PD with an area-under-the-curve of 0.90 and 0.85 on held-out and external test sets, respectively. The AI model can also estimate PD severity and progression in accordance with the Movement Disorder Society Unified Parkinson's Disease Rating Scale (R = 0.94, P = 3.6 × 10-25). The AI model uses an attention layer that allows for interpreting its predictions with respect to sleep and electroencephalogram. Moreover, the model can assess PD in the home setting in a touchless manner, by extracting breathing from radio waves that bounce off a person's body during sleep. Our study demonstrates the feasibility of objective, noninvasive, at-home assessment of PD, and also provides initial evidence that this AI model may be useful for risk assessment before clinical diagnosis.
© 2022. The Author(s).
Conflict of interest statement
C.G.T. has received research support from NINDS and Biosensics. T.D.E. has received research support from the National Institutes of Health, Michael J. Fox Foundation and MedRhythms Inc. T.D.E. has received honorarium for speaking engagements, educational programming and/or outreach activities through the American Parkinson Disease Association, the Parkinson’s Foundation, the Movement Disorders Society and the American Physical Therapy Association. R.D. has received honoraria for speaking at American Academy of Neurology, American Neurological Association, Excellus BlueCross BlueShield, International Parkinson’s and Movement Disorders Society, National Multiple Sclerosis Society, Northwestern University, Physicians Education Resource, LLC, Stanford University, Texas Neurological Society and Weill Cornell; received compensation for consulting services from Abbott, Abbvie, Acadia, Acorda, Alzheimer’s Drug Discovery Foundation, Ascension Health Alliance, Bial-Biotech Investments, Inc., Biogen, BluePrint Orphan, California Pacific Medical Center, Caraway Therapeutics, Clintrex, Curasen Therapeutics, DeciBio, Denali Therapeutics, Eli Lilly, Grand Rounds, Huntington Study Group, medical-legal services, Mediflix, Medopad, Medrhythms, Michael J. Fox Foundation, MJH Holding LLC, NACCME, Neurocrine, NeuroDerm, Olson Research Group, Origent Data Sciences, Otsuka, Pear Therapeutic, Praxis, Prilenia, Roche, Sanofi, Seminal Healthcare, Spark, Springer Healthcare, Sunovion Pharma, Sutter Bay Hospitals, Theravance, University of California Irvine and WebMD; research support from Abbvie, Acadia Pharmaceuticals, Biogen, Biosensics, Burroughs Wellcome Fund, CuraSen, Greater Rochester Health Foundation, Huntington Study Group, Michael J. Fox Foundation, National Institutes of Health, Patient-Centered Outcomes Research Institute, Pfizer, PhotoPharmics, Safra Foundation and Wave Life Sciences; editorial services for Karger Publications; and ownership interests with Grand Rounds (second opinion service). D.K. received research funding from NIH and the Michael J. Fox Foundation. D.K. is a cofounder of Emerald Innovations, Inc., and serves on the scientific advisory board of Janssen and the data and analytics advisory board of Amgen. The remaining authors declare no competing interests.
Figures




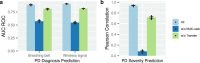
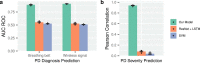

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

