GLP-1-mediated delivery of tesaglitazar improves obesity and glucose metabolism in male mice
- PMID: 35995995
- PMCID: PMC9398908
- DOI: 10.1038/s42255-022-00617-6
GLP-1-mediated delivery of tesaglitazar improves obesity and glucose metabolism in male mice
Abstract
Dual agonists activating the peroxisome proliferator-activated receptors alpha and gamma (PPARɑ/ɣ) have beneficial effects on glucose and lipid metabolism in patients with type 2 diabetes, but their development was discontinued due to potential adverse effects. Here we report the design and preclinical evaluation of a molecule that covalently links the PPARɑ/ɣ dual-agonist tesaglitazar to a GLP-1 receptor agonist (GLP-1RA) to allow for GLP-1R-dependent cellular delivery of tesaglitazar. GLP-1RA/tesaglitazar does not differ from the pharmacokinetically matched GLP-1RA in GLP-1R signalling, but shows GLP-1R-dependent PPARɣ-retinoic acid receptor heterodimerization and enhanced improvements of body weight, food intake and glucose metabolism relative to the GLP-1RA or tesaglitazar alone in obese male mice. The conjugate fails to affect body weight and glucose metabolism in GLP-1R knockout mice and shows preserved effects in obese mice at subthreshold doses for the GLP-1RA and tesaglitazar. Liquid chromatography-mass spectrometry-based proteomics identified PPAR regulated proteins in the hypothalamus that are acutely upregulated by GLP-1RA/tesaglitazar. Our data show that GLP-1RA/tesaglitazar improves glucose control with superior efficacy to the GLP-1RA or tesaglitazar alone and suggest that this conjugate might hold therapeutic value to acutely treat hyperglycaemia and insulin resistance.
© 2022. The Author(s).
Conflict of interest statement
M.H.T. is a member of the scientific advisory board of ERX Pharmaceuticals. He was a member of the Research Cluster Advisory Panel (ReCAP) of the Novo Nordisk Foundation between 2017 and 2019. He attended a scientific advisory board meeting of the Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, in 2016. He received funding for his research projects by Novo Nordisk (2016–2020) and Sanofi-Aventis (2012–2019). He was a consultant for Bionorica SE (2013–2017), Menarini Ricerche S.p.A. (2016), and Bayer Pharma AG Berlin (2016). As former Director of the Helmholtz Diabetes Center and the Institute for Diabetes and Obesity at Helmholtz Zentrum München (2011–2018), and since 2018, as CEO of Helmholtz Zentrum München, he has been responsible for collaborations with a multitude of companies and institutions, worldwide. In this capacity, he discussed potential projects with and has signed/signs contracts for his institute(s) and for the staff for research funding and/or collaborations with industry and academia, worldwide, including but not limited to pharmaceutical corporations such as Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Medigene, Arbormed, BioSyngen and others. In this role, he was/is further responsible for commercial technology transfer activities of his institute(s), including diabetes related patent portfolios of Helmholtz Zentrum München as, for example, WO/2016/188932 A2 or WO/2017/194499 A1. M.H.T. confirms that to the best of his knowledge none of the above funding sources were involved in the preparation of this paper. T.D.M. and K.S. receive research funding by Novo Nordisk. J.E.C. receives research funding from Novo Nordisk and Eli Lilly. D.J.D. has received speaking or consulting fees from Altimmune, Amgen, Eli Lilly, Kallyope, Merck, Novo Nordisk Inc. and Pfizer Inc. Mt. Sinai Hospital receives funding for preclinical studies in the Drucker laboratory from Novo Nordisk and Pfizer Inc. RDDiM is a coinventor on intellectual property owned by Indiana University and licensed to Novo Nordisk. He was previously employed by Novo Nordisk. B.F., B.Y., P.J.K., J.D. and B.D.B. are current employees of Novo Nordisk. The remaining authors declare no competing interests.
Figures



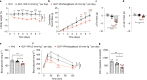



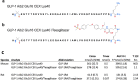



Comment in
-
Who knew? PPARs may act in the brain too.Nat Metab. 2022 Aug;4(8):965-966. doi: 10.1038/s42255-022-00625-6. Nat Metab. 2022. PMID: 35995998 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

