Synergetic Antimicrobial Activity and Mechanism of Clotrimazole-Linked CO-Releasing Molecules
- PMID: 35996473
- PMCID: PMC9389576
- DOI: 10.1021/acsbiomedchemau.2c00007
Synergetic Antimicrobial Activity and Mechanism of Clotrimazole-Linked CO-Releasing Molecules
Abstract
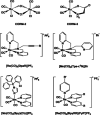
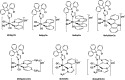
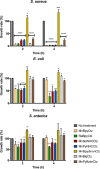
Several metal-based carbon monoxide-releasing molecules (CORMs) are active CO donors with established antibacterial activity. Among them, CORM conjugates with azole antibiotics of type [Mn(CO)3(2,2'-bipyridyl)(azole)]+ display important synergies against several microbes. We carried out a structure-activity relationship study based upon the lead structure of [Mn(CO)3(Bpy)(Ctz)]+ by producing clotrimazole (Ctz) conjugates with varying metal and ligands. We concluded that the nature of the bidentate ligand strongly influences the bactericidal activity, with the substitution of bipyridyl by small bicyclic ligands leading to highly active clotrimazole conjugates. On the contrary, the metal did not influence the activity. We found that conjugate [Re(CO)3(Bpy)(Ctz)]+ is more than the sum of its parts: while precursor [Re(CO)3(Bpy)Br] has no antibacterial activity and clotrimazole shows only moderate minimal inhibitory concentrations, the potency of [Re(CO)3(Bpy)(Ctz)]+ is one order of magnitude higher than that of clotrimazole, and the spectrum of bacterial target species includes Gram-positive and Gram-negative bacteria. The addition of [Re(CO)3(Bpy)(Ctz)]+ to Staphylococcus aureus causes a general impact on the membrane topology, has inhibitory effects on peptidoglycan biosynthesis, and affects energy functions. The mechanism of action of this kind of CORM conjugates involves a sequence of events initiated by membrane insertion, followed by membrane disorganization, inhibition of peptidoglycan synthesis, CO release, and break down of the membrane potential. These results suggest that conjugation of CORMs to known antibiotics may produce useful structures with synergistic effects that increase the conjugate's activity relative to that of the antibiotic alone.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
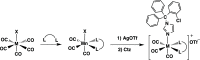


Similar articles
-
Antimicrobial Activity of Manganese(I) Tricarbonyl Complexes Bearing 1,2,3-Triazole Ligands.Molecules. 2023 Nov 6;28(21):7453. doi: 10.3390/molecules28217453. Molecules. 2023. PMID: 37959872 Free PMC article.
-
Conjugated carbon monoxide-releasing molecules have broad-spectrum antimicrobial activity.Future Med Chem. 2023 Jun;15(12):1037-1048. doi: 10.4155/fmc-2023-0103. Epub 2023 Jul 17. Future Med Chem. 2023. PMID: 37458074
-
Examining the antimicrobial activity and toxicity to animal cells of different types of CO-releasing molecules.Dalton Trans. 2016 Jan 28;45(4):1455-66. doi: 10.1039/c5dt02238j. Dalton Trans. 2016. PMID: 26673556
-
Recent Advances on Carbon Monoxide Releasing Molecules for Antibacterial Applications.ChemMedChem. 2021 Dec 14;16(24):3628-3634. doi: 10.1002/cmdc.202100555. Epub 2021 Oct 6. ChemMedChem. 2021. PMID: 34613654 Review.
-
Nonmetallic carbon monoxide releasing molecules (CORMs).Org Biomol Chem. 2017 Oct 25;15(41):8692-8699. doi: 10.1039/c7ob01674c. Org Biomol Chem. 2017. PMID: 28948260 Review.
Cited by
-
Antimicrobial Activity of Rhenium Di- and Tricarbonyl Diimine Complexes: Insights on Membrane-Bound S. aureus Protein Binding.Pharmaceuticals (Basel). 2022 Sep 5;15(9):1107. doi: 10.3390/ph15091107. Pharmaceuticals (Basel). 2022. PMID: 36145328 Free PMC article.
-
Antimicrobial Activity of Manganese(I) Tricarbonyl Complexes Bearing 1,2,3-Triazole Ligands.Molecules. 2023 Nov 6;28(21):7453. doi: 10.3390/molecules28217453. Molecules. 2023. PMID: 37959872 Free PMC article.
-
Clotrimazole-Loaded Borneol-Based In Situ Forming Gel as Oral Sprays for Oropharyngeal Candidiasis Therapy.Gels. 2023 May 15;9(5):412. doi: 10.3390/gels9050412. Gels. 2023. PMID: 37233003 Free PMC article.
-
Computer-Aided Drug Design and Synthesis of Rhenium Clotrimazole Antimicrobial Agents.Antibiotics (Basel). 2023 Mar 20;12(3):619. doi: 10.3390/antibiotics12030619. Antibiotics (Basel). 2023. PMID: 36978486 Free PMC article.
-
A novel clotrimazole selenium nano-composite for combating deep dermal Candida albicans infections and virulence genes.J Antibiot (Tokyo). 2025 Jun;78(7):418-435. doi: 10.1038/s41429-025-00831-w. Epub 2025 May 29. J Antibiot (Tokyo). 2025. PMID: 40442409
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

