Extracellular vesicles in atherothrombosis: From biomarkers and precision medicine to therapeutic targets
- PMID: 35996799
- PMCID: PMC9805233
- DOI: 10.1111/imr.13127
Extracellular vesicles in atherothrombosis: From biomarkers and precision medicine to therapeutic targets
Abstract
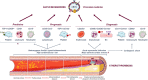
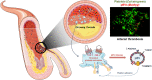
Atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of global mortality. Extracellular vesicles (EVs) are small phospholipid vesicles that convey molecular bioactive cargoes and play essential roles in intercellular communication and, hence, a multifaceted role in health and disease. The present review offers a glimpse into the current state and up-to-date concepts on EV field. It also covers their association with several cardiovascular risk factors and ischemic conditions, being subclinical atherosclerosis of utmost relevance for prevention. Interestingly, we show that EVs hold promise as prognostic and diagnostic as well as predictive markers of ASCVD in the precision medicine era. We then report on the role of EVs in atherothrombosis, disentangling the mechanisms involved in the initiation, progression, and complication of atherosclerosis and showing their direct effect in the context of arterial thrombosis. Finally, their potential use for therapeutic intervention is highlighted.
Keywords: atherosclerosis; extracellular vesicles; inflammation; ischemic disease; microvesicles; thrombosis.
© 2022 The Authors. Immunological Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
L.B. received a research grant from AstraZeneca; hold advisory board work for Sanofi, Pfizer and Novartis; received speaker fees from Bayer, Novartis and Sanofi; and is founder and shareholder of Glycardial Diagnostics SL and Ivestatin Therapeutics SL (all outside of this work). T.P. and G.V. are founders and shareholders of Glycardial Diagnostics SL and Ivestatin Therapeutics (all outside of this work). G.A., M.B‐P., and R.S. have no relevant financial or non‐financial interests to disclose.
Figures

Similar articles
-
Extracellular Vesicles as Drivers of Immunoinflammation in Atherothrombosis.Cells. 2022 Jun 5;11(11):1845. doi: 10.3390/cells11111845. Cells. 2022. PMID: 35681540 Free PMC article. Review.
-
Multifaceted role of extracellular vesicles in atherosclerosis.Atherosclerosis. 2021 Feb;319:121-131. doi: 10.1016/j.atherosclerosis.2020.11.006. Epub 2020 Nov 11. Atherosclerosis. 2021. PMID: 33261815 Review.
-
Extracellular vesicles in atherosclerosis.Clin Chim Acta. 2019 Aug;495:109-117. doi: 10.1016/j.cca.2019.04.051. Epub 2019 Apr 5. Clin Chim Acta. 2019. PMID: 30959044 Review.
-
Extracellular Vesicles: Versatile Nanomediators, Potential Biomarkers and Therapeutic Agents in Atherosclerosis and COVID-19-Related Thrombosis.Int J Mol Sci. 2021 May 31;22(11):5967. doi: 10.3390/ijms22115967. Int J Mol Sci. 2021. PMID: 34073119 Free PMC article. Review.
-
Diversity of extracellular vesicle sources in atherosclerosis: role and therapeutic application.Angiogenesis. 2025 Jun 16;28(3):34. doi: 10.1007/s10456-025-09983-7. Angiogenesis. 2025. PMID: 40522508 Review.
Cited by
-
Immunometabolism in heart failure.Nat Rev Cardiol. 2025 Jun 22. doi: 10.1038/s41569-025-01165-8. Online ahead of print. Nat Rev Cardiol. 2025. PMID: 40544171 Review.
-
Engineering extracellular vesicles for targeted therapeutics in cardiovascular disease.Front Cardiovasc Med. 2024 Dec 19;11:1503830. doi: 10.3389/fcvm.2024.1503830. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39749310 Free PMC article. Review.
-
Extracellular Vesicles as Mediators in Atherosclerotic Cardiovascular Disease.J Lipid Atheroscler. 2024 Sep;13(3):232-261. doi: 10.12997/jla.2024.13.3.232. Epub 2024 Aug 26. J Lipid Atheroscler. 2024. PMID: 39355407 Free PMC article. Review.
-
High Levels of Triggering Receptor Expressed in Myeloid Cells-Like Transcript-1 Positive, but Not Glycoprotein 1b+, Microparticles Are Associated With Poor Outcomes in Acute Respiratory Distress Syndrome.Crit Care Explor. 2024 Jun 27;6(7):e1108. doi: 10.1097/CCE.0000000000001108. eCollection 2024 Jul 1. Crit Care Explor. 2024. PMID: 38935146 Free PMC article.
-
Malondialdehyde-specific natural IgM inhibit NETosis triggered by culprit site-derived extracellular vesicles from myocardial infarction patients.Eur Heart J. 2025 Mar 7;46(10):926-939. doi: 10.1093/eurheartj/ehae584. Eur Heart J. 2025. PMID: 39215577 Free PMC article.
References
-
- van Niel G, Carter DRF, Clayton A, Lambert DW, Raposo G, Vader P. Challenges and directions in studying cell‐cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. 2022;23(5):369‐382. - PubMed
-
- Coly PM, Boulanger CM. Role of extracellular vesicles in atherosclerosis: an update. J Leukoc Biol. 2022;111(1):51‐62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

