Magnetic resonance imaging during warm ex vivo kidney perfusion
- PMID: 35996889
- PMCID: PMC10086841
- DOI: 10.1111/aor.14391
Magnetic resonance imaging during warm ex vivo kidney perfusion
Abstract
Background: The shortage of donor organs for transplantation remains a worldwide problem. The utilization of suboptimal deceased donors enlarges the pool of potential organs, yet consequently, clinicians face the difficult decision of whether these sub-optimal organs are of sufficient quality for transplantation. Novel technologies could play a pivotal role in making pre-transplant organ assessment more objective and reliable.
Methods: Ex vivo normothermic machine perfusion (NMP) at temperatures around 35-37°C allows organ quality assessment in a near-physiological environment. Advanced magnetic resonance imaging (MRI) techniques convey unique information about an organ's structural and functional integrity. The concept of applying magnetic resonance imaging during renal normothermic machine perfusion is novel in both renal and radiological research and we have developed the first MRI-compatible NMP setup for human-sized kidneys.
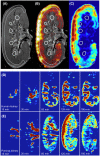
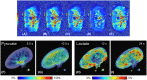
Results: We were able to obtain a detailed and real-time view of ongoing processes inside renal grafts during ex vivo perfusion. This new technique can visualize structural abnormalities, quantify regional flow distribution, renal metabolism, and local oxygen availability, and track the distribution of ex vivo administered cellular therapy.
Conclusion: This platform allows for advanced pre-transplant organ assessment, provides a new realistic tool for studies into renal physiology and metabolism, and may facilitate therapeutic tracing of pharmacological and cellular interventions to an isolated kidney.
Keywords: kidney transplantation; magnetic resonance imaging; renal physiology.
© 2022 The Authors. Artificial Organs published by International Center for Artificial Organ and Transplantation (ICAOT) and Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no competing interests, potential conflicts of interest, or any financial or personal relationship with organizations that could potentially be perceived as influencing the described research.
Figures



References
-
- Rao S, Ghanta M, Moritz MJ, Constantinescu S. Long‐term functional recovery, quality of life, and pregnancy after solid organ transplantation. Med Clin North Am. 2016;100(3):613–29. - PubMed
-
- Seiler A, Klaghofer R, Ture M, Komossa K, Martin‐Soelch C, Jenewein J. A systematic review of health‐related quality of life and psychological outcomes after lung transplantation. J Heart Lung Transplant. 2016;35(2):195–202. - PubMed
-
- Emin A, Rogers CA, Banner NR, Steering Group UKCTA . Quality of life of advanced chronic heart failure: medical care, mechanical circulatory support and transplantation. Eur J Cardiothorac Surg. 2016;50(2):269–73. - PubMed
-
- Rajkumar T, Mazid S, Vucak‐Dzumhur M, Sykes TM, Elder GJ. Health‐related quality of life following kidney and simultaneous pancreas kidney transplantation. Nephrology (Carlton). 2019;24(9):975–82. - PubMed
-
- Schnitzler MA, Lentine KL, Gheorghian A, Axelrod D, Trivedi D, L'Italien G. Renal function following living, standard criteria deceased and expanded criteria deceased donor kidney transplantation: impact on graft failure and death. Transpl Int. 2012;25(2):179–91. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

