Use of breast surveillance between women with pathogenic variants and variants of uncertain significance in breast cancer susceptibility genes
- PMID: 35996941
- PMCID: PMC11160485
- DOI: 10.1002/cncr.34429
Use of breast surveillance between women with pathogenic variants and variants of uncertain significance in breast cancer susceptibility genes
Abstract
Background: Use of surveillance mammography and magnetic resonance imaging (MRI) has been understudied among women with variant of uncertain significance (VUS) compared to pathogenic and likely pathogenic variants (P/LP).
Methods: Using data from two cancer settings, we calculated use of risk-reducing mastectomy (RRM) and surveillance during each 13-month span after genetic testing up to 6 years afterwards for a cohort of genetically elevated risk women.
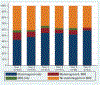
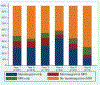
Results: Of 889 women, VUS carriers were less likely to undergo RRM compared to those with P/LP (hazard ratio [HR], 0.17; p = <.001) and high-risk women were more likely to undergo RRM than average-risk women (HR, 3.91; p = .005). Longitudinally, surveillance use among unaffected women decreased from 49.8% in the first year to 31.2% in the sixth year after genetic testing. In comparison, a greater proportion of women with a personal history of breast cancer underwent surveillance, which increased from 59.3% in the first year to 63.6% in the sixth year after genetic testing. Mammography rates did not differ between women with P/LP and VUS within the first 13 months after genetic testing and up to 4 years afterward. Over the first 4 years after genetic testing, women with VUS were less likely to undergo annual MRIs compared to P/LP.
Conclusion: The authors found that VUS, whether in high or moderate penetrance breast cancer susceptibility genes, was associated with lower use of annual breast MRI compared to P/LP variants and equivalent use of annual mammography. These results add important evidence regarding VUS-related breast surveillance.
Keywords: breast; cancer MRI; mammography; surveillance; variant of uncertain significance.
© 2022 American Cancer Society.
Conflict of interest statement
Conflicts of interest: The authors have no relevant financial or non-financial interests to disclose.
Figures
References
-
- Daly MB, Pal T, Berry MP, et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(1):77–102. - PubMed
-
- van Dijk S, Otten W, Timmermans DRM, et al. What’s the message? Interpretation of an uninformative BRCA1/2 test result for women at risk of familial breast cancer. Genetics in Medicine. 2005;7(4):239–245. - PubMed
-
- Ruddy KJ, Sangaralingham L, Freedman RA, et al. Adherence to Guidelines for Breast Surveillance in Breast Cancer Survivors. J Natl Compr Canc Netw. 2018;16(5):526–534. - PubMed
-
- Geller BM, Kerlikowske K, Carney PA, et al. Mammography surveillance following breast cancer. Breast Cancer Res Treat. 2003;81(2):107–115. - PubMed
-
- Peshkin BN, Schwartz MD, Isaacs C, Hughes C, Main D, Lerman C. Utilization of Breast Cancer Screening in a Clinically Based Sample of Women after <strong>BRCA1/2</strong> Testing. Cancer Epidemiology Biomarkers & Prevention . 2002;11(10):1115–1118. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

