Epipolythiodioxopiperazine-Based Natural Products: Building Blocks, Biosynthesis and Biological Activities
- PMID: 35997236
- PMCID: PMC10086836
- DOI: 10.1002/cbic.202200341
Epipolythiodioxopiperazine-Based Natural Products: Building Blocks, Biosynthesis and Biological Activities
Abstract
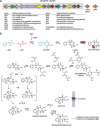
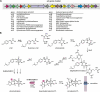
Epipolythiodioxopiperazines (ETPs) are fungal secondary metabolites that share a 2,5-diketopiperazine scaffold built from two amino acids and bridged by a sulfide moiety. Modifications of the core and the amino acid side chains, for example by methylations, acetylations, hydroxylations, prenylations, halogenations, cyclizations, and truncations create the structural diversity of ETPs and contribute to their biological activity. However, the key feature responsible for the bioactivities of ETPs is their sulfide moiety. Over the last years, combinations of genome mining, reverse genetics, metabolomics, biochemistry, and structural biology deciphered principles of ETP production. Sulfurization via glutathione and uncovering of the thiols followed by either oxidation or methylation crystallized as fundamental steps that impact expression of the biosynthesis cluster, toxicity and secretion of the metabolite as well as self-tolerance of the producer. This article showcases structure and activity of prototype ETPs such as gliotoxin and discusses the current knowledge on the biosynthesis routes of these exceptional natural products.
Keywords: biosynthetic gene clusters; disulfide bridges; enzymatic reactions; fungi; toxins.
© 2022 The Authors. ChemBioChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

