Adjuvant Therapy After Neoadjuvant Therapy for Esophageal Cancer: Who Needs It?
- PMID: 35997269
- PMCID: PMC10955553
- DOI: 10.1097/SLA.0000000000005679
Adjuvant Therapy After Neoadjuvant Therapy for Esophageal Cancer: Who Needs It?
Abstract
Objective: We hypothesized that, on average, patients do not benefit from additional adjuvant therapy after neoadjuvant therapy for locally advanced esophageal cancer, although subsets of patients might. Therefore, we sought to identify profiles of patients predicted to receive the most survival benefit or greatest detriment from adding adjuvant therapy.
Background: Although neoadjuvant therapy has become the treatment of choice for locally advanced esophageal cancer, the value of adding adjuvant therapy is unknown.
Methods: From 1970 to 2014, 22,123 patients were treated for esophageal cancer at 33 centers on 6 continents (Worldwide Esophageal Cancer Collaboration), of whom 7731 with adenocarcinoma or squamous cell carcinoma received neoadjuvant therapy; 1348 received additional adjuvant therapy. Random forests for survival and virtual-twin analyses were performed for all-cause mortality.
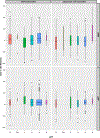
Results: Patients received a small survival benefit from adjuvant therapy (3.2±10 months over the subsequent 10 years for adenocarcinoma, 1.8±11 for squamous cell carcinoma). Consistent benefit occurred in ypT3-4 patients without nodal involvement and those with ypN2-3 disease. The small subset of patients receiving most benefit had high nodal burden, ypT4, and positive margins. Patients with ypT1-2N0 cancers had either no benefit or a detriment in survival.
Conclusions: Adjuvant therapy after neoadjuvant therapy has value primarily for patients with more advanced esophageal cancer. Because the benefit is often small, patients considering adjuvant therapy should be counseled on benefits versus morbidity. In addition, given that the overall benefit was meaningful in a small number of patients, emerging modalities such as immunotherapy may hold more promise in the adjuvant setting.
Copyright © 2022 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

References
-
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. - PubMed
-
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. - PubMed
-
- Klevebro F, Alexandersson von Dobeln G, Wang N, et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol. 2016;27:660–667. - PubMed
-
- Burmeister BH, Thomas JM, Burmeister EA, et al. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer. 2011;47:354–360. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

