Early initiation of short-term emollient use for the prevention of atopic dermatitis in high-risk infants-The STOP-AD randomised controlled trial
- PMID: 35997592
- PMCID: PMC10952678
- DOI: 10.1111/all.15491
Early initiation of short-term emollient use for the prevention of atopic dermatitis in high-risk infants-The STOP-AD randomised controlled trial
Abstract
Background: Protecting the skin barrier in early infancy may prevent atopic dermatitis (AD). We investigated if daily emollient use from birth to 2 months reduced AD incidence in high-risk infants at 12 months.
Methods: This was a single-center, two-armed, investigator-blinded, randomized controlled clinical trial (NCT03871998). Term infants identified as high risk for AD (parental history of AD, asthma or allergic rhinitis) were recruited within 4 days of birth and randomised 1:1 to either twice-daily emollient application for the first 8 weeks of life (intervention group), using an emollient specifically formulated for very dry, AD-prone skin, or to standard routine skin care (control group). The primary outcome was cumulative AD incidence at 12 months. AD <6 months was diagnosed based on clinical presence of AD. The UK Working Party Diagnostic Criteria were applied when diagnosing AD between 6 and 12 months.
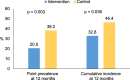
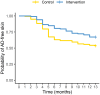
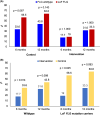
Results: Three hundred twenty-one were randomised (161 intervention and 160 control), with 61 withdrawals (41 intervention, 20 control). The cumulative incidence of AD at 12 months was 32.8% in the intervention group vs. 46.4% in the control group, p = 0.036 [Relative risk (95%CI): 0.707 (0.516, 0.965)]. One infant in the intervention group was withdrawn from the study following development of a rash that had a potential relationship with the emollient. There was no significant difference in the incidence of skin infections between the intervention and control groups during the intervention period (5.0% vs. 5.7%, p > 0.05).
Conclusions: This study has demonstrated that early initiation of daily specialized emollient use until 2 months reduces the incidence of AD in the first year of life in high-risk infants.
Keywords: atopic dermatitis; emollient; prevention; randomized controlled trial; skin barrier.
© 2022 The Authors. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
The emollient used in this study was provided by its manufacturer Johnson and Johnson Santé Beauté France (JJSBF) as benefit‐in‐kind. JJSBF did not have any role in the trial design. JJSBF provided funding for a sub‐study within this study, which includes the analysis of the microbiome and inflammatory biomarkers in a subgroup of the study. This is separate from the study's primary funding source. CNC, DL, DM, XFCCW, and JEC declare no conflicts of interest. CN and GJP are employees of RiverD International B.V., which produced the NMF‐scan. GJP is the managing director and a shareholder of RiverD International B.V. JO'BH receives research funding, speaker fees, and consultancy fees from Aimmune Therapeutics, research funding and speaker fees from DBV Technologies, and research funding from Clemens von Pirquet Foundation and Temple St Hospital Foundation. ADI is a consultant/on the advisory board for AbbVie, Novartis, Regeneron, Sanofi Genzyme, Pfizer, Eli Lilly, Amgen, Benevolent AI, LEO, Arena. A patent application has been submitted by Johnson & Johnson based on these results, with JO'BH and ADI named as inventors.
Figures

Comment in
-
Editorial comments on "Early initiation of short-term emollient use for the prevention of atopic dermatitis in high-risk infants-The STOP-AD randomized controlled trial"-Is emollient therapy enough?Allergy. 2023 Apr;78(4):908-911. doi: 10.1111/all.15626. Allergy. 2023. PMID: 36573442 No abstract available.
-
Food allergens in emollients.Allergy. 2023 Apr;78(4):1121-1122. doi: 10.1111/all.15546. Allergy. 2023. PMID: 37002656 No abstract available.
-
Reply to correspondence "Food allergens to emollients".Allergy. 2023 Apr;78(4):1123. doi: 10.1111/all.15651. Allergy. 2023. PMID: 37002657 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

