Monocyte-derived macrophages aggravate pulmonary vasculitis via cGAS/STING/IFN-mediated nucleic acid sensing
- PMID: 35997679
- PMCID: PMC9402992
- DOI: 10.1084/jem.20220759
Monocyte-derived macrophages aggravate pulmonary vasculitis via cGAS/STING/IFN-mediated nucleic acid sensing
Erratum in
-
Correction: Monocyte-derived macrophages aggravate pulmonary vasculitis via cGAS/STING/IFN-mediated nucleic acid sensing.J Exp Med. 2022 Dec 5;219(12):e2022075911022022c. doi: 10.1084/jem.2022075911022022c. Epub 2022 Nov 11. J Exp Med. 2022. PMID: 36367753 Free PMC article. No abstract available.
Abstract
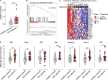
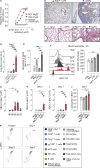
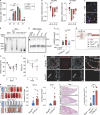
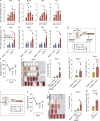
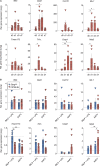
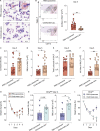
Autoimmune vasculitis is a group of life-threatening diseases, whose underlying pathogenic mechanisms are incompletely understood, hampering development of targeted therapies. Here, we demonstrate that patients suffering from anti-neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (AAV) showed increased levels of cGAMP and enhanced IFN-I signature. To identify disease mechanisms and potential therapeutic targets, we developed a mouse model for pulmonary AAV that mimics severe disease in patients. Immunogenic DNA accumulated during disease onset, triggering cGAS/STING/IRF3-dependent IFN-I release that promoted endothelial damage, pulmonary hemorrhages, and lung dysfunction. Macrophage subsets played dichotomic roles in disease. While recruited monocyte-derived macrophages were major disease drivers by producing most IFN-β, resident alveolar macrophages contributed to tissue homeostasis by clearing red blood cells and limiting infiltration of IFN-β-producing macrophages. Moreover, pharmacological inhibition of STING, IFNAR-I, or its downstream JAK/STAT signaling reduced disease severity and accelerated recovery. Our study unveils the importance of STING/IFN-I axis in promoting pulmonary AAV progression and identifies cellular and molecular targets to ameliorate disease outcomes.
© 2022 Kessler et al.
Conflict of interest statement
Disclosures: S.F. Viehmann started a job at AstraZeneca GmbH in the respiratory and immunology department after writing the original draft. At this time, research contributions submitted for publication were completed. R. Behrendt reported grants from Hoffmann-La Roche, ISD Immunotech, and IFM Therapeutics outside the submitted work. A. Ablasser is a co-founder of IFM-Due. P.A. Lyons reported grants from Medical Research Council during the conduct of the study; and holds founding equity in and consult for PredictImmune Ltd. No other disclosures were reported.
Figures






References
-
- Bain, C.C., Scott C.L., Uronen-Hansson H., Gudjonsson S., Jansson O., Grip O., Guilliams M., Malissen B., Agace W.W., and Mowat A.M.. 2013September. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 6:498–510. 10.1038/mi.2012.89 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

