Effect of Aspirin vs Enoxaparin on Symptomatic Venous Thromboembolism in Patients Undergoing Hip or Knee Arthroplasty: The CRISTAL Randomized Trial
- PMID: 35997730
- PMCID: PMC9399863
- DOI: 10.1001/jama.2022.13416
Effect of Aspirin vs Enoxaparin on Symptomatic Venous Thromboembolism in Patients Undergoing Hip or Knee Arthroplasty: The CRISTAL Randomized Trial
Abstract
Importance: There remains a lack of randomized trials investigating aspirin monotherapy for symptomatic venous thromboembolism (VTE) prophylaxis following total hip arthroplasty (THA) or total knee arthroplasty (TKA).
Objective: To determine whether aspirin was noninferior to enoxaparin in preventing symptomatic VTE after THA or TKA.
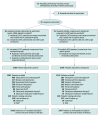
Design, setting, and participants: Cluster-randomized, crossover, registry-nested trial across 31 hospitals in Australia. Clusters were hospitals performing greater than 250 THA or TKA procedures annually. Patients (aged ≥18 years) undergoing hip or knee arthroplasty procedures were enrolled at each hospital. Patients receiving preoperative anticoagulation or who had a medical contraindication to either study drug were excluded. A total of 9711 eligible patients were enrolled (5675 in the aspirin group and 4036 in the enoxaparin group) between April 20, 2019, and December 18, 2020. Final follow-up occurred on August 14, 2021.
Interventions: Hospitals were randomized to administer aspirin (100 mg/d) or enoxaparin (40 mg/d) for 35 days after THA and for 14 days after TKA. Crossover occurred after the patient enrollment target had been met for the first group. All 31 hospitals were initially randomized and 16 crossed over prior to trial cessation.
Main outcomes and measures: The primary outcome was symptomatic VTE within 90 days, including pulmonary embolism and deep venous thrombosis (DVT) (above or below the knee). The noninferiority margin was 1%. Six secondary outcomes are reported, including death and major bleeding within 90 days. Analyses were performed by randomization group.
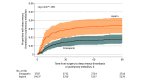
Results: Enrollment was stopped after an interim analysis determined the stopping rule was met, with 9711 patients (median age, 68 years; 56.8% female) of the prespecified 15 562 enrolled (62%). Of these, 9203 (95%) completed the trial. Within 90 days of surgery, symptomatic VTE occurred in 256 patients, including pulmonary embolism (79 cases), above-knee DVT (18 cases), and below-knee DVT (174 cases). The symptomatic VTE rate in the aspirin group was 3.45% and in the enoxaparin group was 1.82% (estimated difference, 1.97%; 95% CI, 0.54%-3.41%). This failed to meet the criterion for noninferiority for aspirin and was significantly superior for enoxaparin (P = .007). Of 6 secondary outcomes, none were significantly better in the enoxaparin group compared with the aspirin group.
Conclusions and relevance: Among patients undergoing hip or knee arthroplasty for osteoarthritis, aspirin compared with enoxaparin resulted in a significantly higher rate of symptomatic VTE within 90 days, defined as below- or above-knee DVT or pulmonary embolism. These findings may be informed by a cost-effectiveness analysis.
Trial registration: ANZCTR Identifier: ACTRN12618001879257.
Conflict of interest statement
Figures

Comment in
-
Thromboprophylaxis After Hip or Knee Arthroplasty.JAMA. 2022 Aug 23;328(8):712-713. doi: 10.1001/jama.2022.11249. JAMA. 2022. PMID: 35997752 No abstract available.
-
Review of Article: CRISTAL Study Group. Effect of aspirin vs. enoxaparin on symptomatic venous thromboembolism in patients undergoing hip or knee arthroplasty: The CRISTAL randomized trial. JAMA. 2022;328(8):719-727.J Vasc Nurs. 2022 Sep;40(3):155-156. doi: 10.1016/j.jvn.2022.10.001. J Vasc Nurs. 2022. PMID: 36414372 No abstract available.
-
In THA or TKA, risk for symptomatic VTE was higher with aspirin vs. enoxaparin at 90 d.Ann Intern Med. 2022 Dec;175(12):JC141. doi: 10.7326/J22-0096. Epub 2022 Dec 6. Ann Intern Med. 2022. PMID: 36469926
-
Low-Molecular-Weight Heparin Is Superior to Aspirin in the Prevention of Thromboembolic Disease: Or Is It?J Bone Joint Surg Am. 2022 Dec 7;104(23):2045-2046. doi: 10.2106/JBJS.22.01024. Epub 2022 Nov 4. J Bone Joint Surg Am. 2022. PMID: 36476736 No abstract available.
-
Aspirin vs Enoxaparin and Symptomatic Venous Thromboembolism in Hip or Knee Arthroplasty.JAMA. 2023 Jan 10;329(2):176-177. doi: 10.1001/jama.2022.20751. JAMA. 2023. PMID: 36625814 No abstract available.
-
Aspirin vs Enoxaparin and Symptomatic Venous Thromboembolism in Hip or Knee Arthroplasty.JAMA. 2023 Jan 10;329(2):176. doi: 10.1001/jama.2022.20748. JAMA. 2023. PMID: 36625815 No abstract available.
-
Aspirin vs Enoxaparin and Symptomatic Venous Thromboembolism in Hip or Knee Arthroplasty.JAMA. 2023 Jan 10;329(2):177. doi: 10.1001/jama.2022.20745. JAMA. 2023. PMID: 36625816 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

