The pulmonary vasculature in lethal COVID-19 and idiopathic pulmonary fibrosis at single-cell resolution
- PMID: 35998078
- PMCID: PMC9452154
- DOI: 10.1093/cvr/cvac139
The pulmonary vasculature in lethal COVID-19 and idiopathic pulmonary fibrosis at single-cell resolution
Abstract
Aims: Severe acute respiratory syndrome coronavirus-2 infection causes COVID-19, which in severe cases evokes life-threatening acute respiratory distress syndrome (ARDS). Transcriptome signatures and the functional relevance of non-vascular cell types (e.g. immune and epithelial cells) in COVID-19 are becoming increasingly evident. However, despite its known contribution to vascular inflammation, recruitment/invasion of immune cells, vascular leakage, and perturbed haemostasis in the lungs of severe COVID-19 patients, an in-depth interrogation of the endothelial cell (EC) compartment in lethal COVID-19 is lacking. Moreover, progressive fibrotic lung disease represents one of the complications of COVID-19 pneumonia and ARDS. Analogous features between idiopathic pulmonary fibrosis (IPF) and COVID-19 suggest partial similarities in their pathophysiology, yet, a head-to-head comparison of pulmonary cell transcriptomes between both conditions has not been implemented to date.
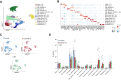
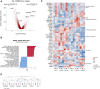
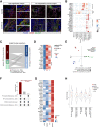
Methods and results: We performed single-nucleus RNA-sequencing on frozen lungs from 7 deceased COVID-19 patients, 6 IPF explant lungs, and 12 controls. The vascular fraction, comprising 38 794 nuclei, could be subclustered into 14 distinct EC subtypes. Non-vascular cell types, comprising 137 746 nuclei, were subclustered and used for EC-interactome analyses. Pulmonary ECs of deceased COVID-19 patients showed an enrichment of genes involved in cellular stress, as well as signatures suggestive of dampened immunomodulation and impaired vessel wall integrity. In addition, increased abundance of a population of systemic capillary and venous ECs was identified in COVID-19 and IPF. COVID-19 systemic ECs closely resembled their IPF counterparts, and a set of 30 genes was found congruently enriched in systemic ECs across studies. Receptor-ligand interaction analysis of ECs with non-vascular cell types in the pulmonary micro-environment revealed numerous previously unknown interactions specifically enriched/depleted in COVID-19 and/or IPF.
Conclusions: This study uncovered novel insights into the abundance, expression patterns, and interactomes of EC subtypes in COVID-19 and IPF, relevant for future investigations into the progression and treatment of both lethal conditions.
Keywords: COVID-19; Endothelial cells; IPF; Lung; SARS-CoV-2; Single-nucleus RNA-seq; Transcriptomics.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: A.D. received payments from FMC Belgium, and has a leadership/fiduciary role in the Belgian Society of Pathology (non-profit), and European Society of Pathology Nephropathology working group (non-profit). B.T. has a consulting role for ONO pharmaceutical and owns 10X genomics stocks. B.W. received payments from Hologic. L.J.C. received a research grant and consulting fees from MEDTRONIC. S.F. received support from Pfizer for congress attendance. W.W. received research grants and payment for lectures from Roche and Boehringer Ingelheim and a research grant from Galapagos. S.V. received consulting fees from Therakos and Boehringer Ingelheim. J.W. received investigator-initiated grants, consulting fees, speaker fees, and travel grants from Pfizer and Gilead.
Figures




References
-
- Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Cheng L, Li J, Wang X, Wang F, Liu L, Amit I, Zhang S, Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 2020;26:842–844. - PubMed
-
- Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martinez-Colon GJ, McKechnie JL, Ivison GT, Ranganath T, Vergara R, Hollis T, Simpson LJ, Grant P, Subramanian A, Rogers AJ, Blish CA. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med 2020;26:1070–1076. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

