Squalene in oil-based adjuvant improves the immunogenicity of SARS-CoV-2 RBD and confirms safety in animal models
- PMID: 35998134
- PMCID: PMC9397949
- DOI: 10.1371/journal.pone.0269823
Squalene in oil-based adjuvant improves the immunogenicity of SARS-CoV-2 RBD and confirms safety in animal models
Abstract
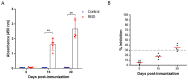
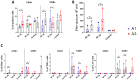
COVID-19 pandemic has accelerated the development of vaccines against its etiologic agent, SARS-CoV-2. However, the emergence of new variants of the virus lead to the generation of new alternatives to improve the current sub-unit vaccines in development. In the present report, the immunogenicity of the Spike RBD of SARS-CoV-2 formulated with an oil-in-water emulsion and a water-in-oil emulsion with squalene was evaluated in mice and hamsters. The RBD protein was expressed in insect cells and purified by chromatography until >95% purity. The protein was shown to have the appropriate folding as determined by ELISA and flow cytometry binding assays to its receptor, as well as by its detection by hamster immune anti-S1 sera under non-reducing conditions. In immunization assays, although the cellular immune response elicited by both adjuvants were similar, the formulation based in water-in-oil emulsion and squalene generated an earlier humoral response as determined by ELISA. Similarly, this formulation was able to stimulate neutralizing antibodies in hamsters. The vaccine candidate was shown to be safe, as demonstrated by the histopathological analysis in lungs, liver and kidney. These results have shown the potential of this formulation vaccine to be evaluated in a challenge against SARS-CoV-2 and determine its ability to confer protection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, et al.. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol 2020 176. 2020;17: 613–620. doi: 10.1038/s41423-020-0400-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

