Evaluation of a rapid semiquantitative lateral flow assay for the prediction of serum neutralizing activity against SARS-CoV-2 variants
- PMID: 35998394
- PMCID: PMC9383946
- DOI: 10.1016/j.jcv.2022.105268
Evaluation of a rapid semiquantitative lateral flow assay for the prediction of serum neutralizing activity against SARS-CoV-2 variants
Abstract
Background: Neutralizing antibodies (NAbs) against SARS-CoV-2 have been shown to correlate with protection against infection. Simple tools such as lateral flow assays (LFA) that can accurately measure NAbs may be useful for monitoring anti-SARS-CoV-2 immunity in the future.
Objectives: We assessed the performance of the ichroma™ COVID-19 nAb test, a rapid semiquantitative LFA, for the prediction of serum neutralizing activity against SARS-CoV-2 variants.
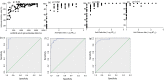
Study design: Serum samples were collected from COVID-19 recovered patients and vaccinated individuals. The result of the ichroma assay was provided as inhibition rate, and was compared to anti-SARS-CoV-2 IgG levels, and NAbs against Alpha, Delta and Omicron variants.
Results: A total of 90 sera from recovered unvaccinated patients and 209 sera from the vaccine cohort were included in this study. In post-infection samples, the ichroma inhbition rate was found to be correlated with IgG levels (ρ = 0.83), and with anti-Alpha NAbs levels (ρ = 0.78). In the vaccine cohort, a good correlation was also observed between the ichroma inhibition rate and IgG levels (ρ = 0.84), as well as NAbs against Alpha (ρ = 0.62), Delta (ρ = 0.88) and Omicron (ρ = 0.74). An ichroma inhbition rate of 77.2%, 90.8% and 99.6% accurately predicted neutralization against Alpha, Delta and Omicron variants respectively.
Conclusions: The ichroma™ COVID-19 nAb assay, with appropriate variant cut-offs, can be useful for the monitoring of anti-SARS-CoV-2 immunization and may provide a rapid prediction of protection, especially in individuals with significant levels of NAbs.
Keywords: Lateral flow assay; Neutralizing antibodies; SARS-CoV-2; Variants.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
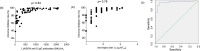
References
-
- Libster R., Pérez Marc G., Wappner D., Coviello S., Bianchi A., Braem V., Esteban I., Caballero M.T., Wood C., Berrueta M., Rondan A., Lescano G., Cruz P., Ritou Y., Fernández Viña V., Álvarez Paggi D., Esperante S., Ferreti A., Ofman G., Ciganda Á., Rodriguez R., Lantos J., Valentini R., Itcovici N., Hintze A., Oyarvide M.L., Etchegaray C., Neira A., Name I., Alfonso J., López Castelo R., Caruso G., Rapelius S., Alvez F., Etchenique F., Dimase F., Alvarez D., Aranda S.S., Sánchez Yanotti C., De Luca J., Jares Baglivo S., Laudanno S., Nowogrodzki F., Larrea R., Silveyra M., Leberzstein G., Debonis A., Molinos J., González M., Perez E., Kreplak N., Pastor Argüello S., Gibbons L., Althabe F., Bergel E., Polack F.P. Fundación INFANT–COVID-19 group, early high-titer plasma therapy to prevent severe Covid-19 in older adults. N. Engl. J. Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. - DOI - PMC - PubMed
-
- Saker K., Escuret V., Pitiot V., Massardier-Pilonchéry A., Paul S., Mokdad B., Langlois-Jacques C., Rabilloud M., Goncalves D., Fabien N., Guibert N., Fassier J.-.B., Bal A., Trouillet-Assant S., Trabaud M.-.A. Evaluation of commercial anti-SARS-CoV-2 antibody assays and comparison of standardized titers in vaccinated health care workers. J. Clin. Microbiol. 2022;60 doi: 10.1128/JCM.01746-21. - DOI - PMC - PubMed
-
- Sander I., Kespohl S., Zahradnik E., Göcke P., Hosbach I., Herrmann B.L., Brüning T., Raulf M. Quantitative measurement of IgG to SARS-CoV-2 antigens using monoclonal antibody-based enzyme-linked immunosorbent assays. Clin. Transl. Immunol. 2022;11:e1369. doi: 10.1002/cti2.1369. - DOI - PMC - PubMed
-
- Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

