Barriers to the Intestinal Absorption of Four Insulin-Loaded Arginine-Rich Nanoparticles in Human and Rat
- PMID: 35998570
- PMCID: PMC9527806
- DOI: 10.1021/acsnano.2c04330
Barriers to the Intestinal Absorption of Four Insulin-Loaded Arginine-Rich Nanoparticles in Human and Rat
Abstract
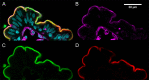
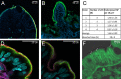
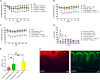
Peptide drugs and biologics provide opportunities for treatments of many diseases. However, due to their poor stability and permeability in the gastrointestinal tract, the oral bioavailability of peptide drugs is negligible. Nanoparticle formulations have been proposed to circumvent these hurdles, but systemic exposure of orally administered peptide drugs has remained elusive. In this study, we investigated the absorption mechanisms of four insulin-loaded arginine-rich nanoparticles displaying differing composition and surface characteristics, developed within the pan-European consortium TRANS-INT. The transport mechanisms and major barriers to nanoparticle permeability were investigated in freshly isolated human jejunal tissue. Cytokine release profiles and standard toxicity markers indicated that the nanoparticles were nontoxic. Three out of four nanoparticles displayed pronounced binding to the mucus layer and did not reach the epithelium. One nanoparticle composed of a mucus inert shell and cell-penetrating octarginine (ENCP), showed significant uptake by the intestinal epithelium corresponding to 28 ± 9% of the administered nanoparticle dose, as determined by super-resolution microscopy. Only a small fraction of nanoparticles taken up by epithelia went on to be transcytosed via a dynamin-dependent process. In situ studies in intact rat jejunal loops confirmed the results from human tissue regarding mucus binding, epithelial uptake, and negligible insulin bioavailability. In conclusion, while none of the four arginine-rich nanoparticles supported systemic insulin delivery, ENCP displayed a consistently high uptake along the intestinal villi. It is proposed that ENCP should be further investigated for local delivery of therapeutics to the intestinal mucosa.
Keywords: human; insulin; jejunum; nanoparticle; oral peptide delivery.
Conflict of interest statement
The authors declare no competing financial interest.
Figures