Safety and immunogenicity of anti-SARS-CoV-2 heterologous scheme with SOBERANA 02 and SOBERANA Plus vaccines: Phase IIb clinical trial in adults
- PMID: 35998623
- PMCID: PMC9359498
- DOI: 10.1016/j.medj.2022.08.001
Safety and immunogenicity of anti-SARS-CoV-2 heterologous scheme with SOBERANA 02 and SOBERANA Plus vaccines: Phase IIb clinical trial in adults
Abstract
Background: SOBERANA 02 has been evaluated in phase I and IIa studies comparing homologous versus heterologous schedule (this one, including SOBERANA Plus). Here, we report results of immunogenicity, safety, and reactogenicity of SOBERANA 02 in a two- or three-dose heterologous scheme in adults.
Method: Phase IIb was a parallel, multicenter, adaptive, double-blind, randomized, and placebo-controlled trial. Subjects (n = 810) aged 19-80 years were randomized to receive two doses of SARS-CoV-2 RBD conjugated to tetanus toxoid (SOBERANA 02) and a third dose of dimeric RBD (SOBERANA Plus) 28 days apart; two production batches of active ingredients of SOBERANA 02 were evaluated. Primary outcome was the percentage of seroconverted subjects with ≥4-fold the anti-RBD immunoglobulin G (IgG) concentration. Secondary outcomes were safety, reactogenicity, and neutralizing antibodies.
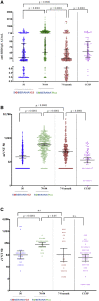
Findings: Seroconversion rate in vaccinees was 76.3% after two doses and 96.8% after the third dose of SOBERANA Plus (7.3% in the placebo group). Neutralizing IgG antibodies were detected against D614G and variants of concern (VOCs) Alpha, Beta, Delta, and Omicron. Specific, functional antibodies were detected 7-8 months after the third dose. The frequency of serious adverse events (AEs) associated with vaccination was very low (0.1%). Local pain was the most frequent AE.
Conclusions: Two doses of SOBERANA 02 were safe and immunogenic in adults. The heterologous combination with SOBERANA Plus increased neutralizing antibodies, detectable 7-8 months after the third dose.
Trial registry: https://rpcec.sld.cu/trials/RPCEC00000347 FUNDING: This work was supported by Finlay Vaccine Institute, BioCubaFarma, and the Fondo Nacional de Ciencia y Técnica (FONCI-CITMA-Cuba, contract 2020-20).
Keywords: COVID-19; Translation to patients; conjugate vaccine; heterologous schedule; phase IIb clinical trial; recombinant RBD.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.E.T.-R., M.G.-C., L.V.-S., S.P.-R., C.V.-S., M.T.P.-G., J.E.-P., E.N.-R., A.P.-D., G.B.-R., I.M.-H., Y.M., Y.G.-M., B.L.S.-V., G.-W.-C., D.D., A.B., and D.G.R. declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. M.R.-G., B.P.-M., B.S.-R., R.G.-M., T.H.-G., I.O.-V., M.D.-H., S.F.-C., Y.C.-R., L.R.-N., D.S.-M., Y.G.-V., T.B.-A., Y.V.-B., D.G.-R., and V.V.-B. work at Finlay Vaccine Institute or the Center of Molecular Immunology, institutions that develop and manufacture the vaccine candidates, but they have not received an honorarium for this paper. B.S.-R., S.F.-C., Y.C.-R., L.R.-N., D.S.-M., Y.V.-B., D.G.R., D.G.-R., and V.V.-B. have filed patent applications related to the vaccine SOBERANA 02.
Figures




References
-
- Regulatory Affairs Professionals Society (RAPS) 2021. COVID-19 Vaccine Tracker.https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vac... Available at:
-
- Valdes-Balbin Y., Santana-Mederos D., Paquet F., Fernandez S., Climent Y., Chiodo F., Rodríguez L., Sanchez-Ramirez B., Leon K., Hernandez T., et al. Molecular aspects concerning the use of the SARS-CoV-2 receptor binding domain as a target for preventive vaccines. ACS Cent. Sci. 2021;7:757–767. doi: 10.1021/acscentsci.1c00216. - DOI - PMC - PubMed
-
- Valdes-Balbin Y., Santana-Mederos D., Quintero L., Fernández S., Rodríguez L., Sanchez-Ramirez B., Perez-Nicado R., Acosta C., Méndez Y., Ricardo M.G., et al. SARS-CoV-2 RBD-Tetanus toxoid conjugate vaccine induces a strong neutralizing immunity. ACS Chem. Biol. 2021;16:1223–1233. doi: 10.1021/acschembio.1c00272. - DOI - PubMed
-
- International Clinical Trials Registry Platform Identifier RPCEC00000340. Phase I study, open, sequential and adaptive for evaluating the safety, reactogenicity and explore the immunogenicity of the prophylactic Vaccine candidate FINLAY-FR-2 anti SARSCoV-2 (COVID-19) https://rpcec.sld.cu/en/trials/RPCEC00000340-En
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

