Off-the-shelf cryopreserved platelets for the detection of HIT and VITT antibodies
- PMID: 35998675
- PMCID: PMC9837435
- DOI: 10.1182/blood.2022017283
Off-the-shelf cryopreserved platelets for the detection of HIT and VITT antibodies
Abstract
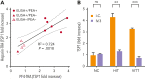
Heparin-induced thrombocytopenia (HIT) is suspected much more often than it is confirmed. Technically simple platelet factor 4 (PF4)-polyanion enzyme-linked immunosorbent assays (ELISAs) are sensitive but nonspecific. In contrast, accurate functional tests such as the serotonin release assay, heparin-induced platelet activation assay, and PF4-dependent P-selectin expression assay require fresh platelets and have complex assay end points, limiting their availability to specialized reference laboratories. To enable broad deployment of functional testing, we sought to extend platelet viability significantly by optimizing storage conditions and developed a simple functional assay end point by measuring the release of a platelet α-granule protein, thrombospondin-1 (TSP1), in an ELISA format. Platelet cryopreservation conditions were optimized by freezing platelets at controlled cooling rates that preserve activatability. Several-month-old cryopreserved platelets were treated with PF4 or heparin and were evaluated for their ability to be activated by HIT and vaccine-induced immune thrombotic thrombocytopenia (VITT) antibodies in the TSP1 release assay (TRA). HIT and spontaneous HIT patient samples induced significantly higher TSP1 release using both PF4-treated (PF4-TRA) and heparin-treated cryopreserved platelets relative to samples from patients suspected of HIT who lacked platelet-activating antibodies. This latter group included several patients that tested strongly positive in PF4-polyanion ELISA but were not platelet-activating. Four VITT patient samples tested in the TRA activated PF4-treated, but not heparin-treated, cryopreserved platelets, consistent with recent data suggesting the requirement for PF4-treated platelets for VITT antibody detection. These findings have the potential to transform the testing paradigm in HIT and VITT, making decentralized, technically simple functional testing available for rapid and accurate in-hospital diagnosis.
© 2022 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: C.G.J. reports pending or issued patents (Versiti Blood Center of Wisconsin and Retham Technologies) and reports equity ownership and employment in Retham Technologies. R.K.P. reports honoraria for advisory board participation from CSL Behring, Genentech, Bayer Healthcare AG, HEMA Biologics, Instrumentation Laboratory, and Merck. G.D.W. receives honoraria for advisory board participation from Diagnostica Stago. A.P. reports pending or issued patents (Mayo Clinic, Retham Technologies, and Versiti BloodCenter of Wisconsin), equity ownership in and serving as an officer of Retham Technologies, and member of the advisory board of Veralox Therapeutics. The remaining authors declare no competing financial interests.
Figures



Comment in
-
A vision for a quicker definitive diagnosis of HIT.Blood. 2022 Dec 22;140(25):2657-2658. doi: 10.1182/blood.2022018158. Blood. 2022. PMID: 36548019 No abstract available.
References
-
- Greinacher A. Heparin-induced thrombocytopenia. N Engl J Med. 2015;373(19):1883–1884. - PubMed
-
- Warkentin TE. Laboratory diagnosis of heparin-induced thrombocytopenia. Int J Lab Hematol. 2019;41(suppl 1):15–25. - PubMed
-
- Cuker A. Clinical and laboratory diagnosis of heparin-induced thrombocytopenia: an integrated approach. Semin Thromb Hemost. 2014;40(1):106–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

