Acid-Base Disorders in the Critically Ill Patient
- PMID: 35998977
- PMCID: PMC10101555
- DOI: 10.2215/CJN.04500422
Acid-Base Disorders in the Critically Ill Patient
Abstract
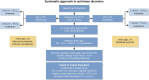
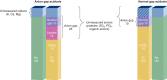
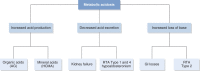
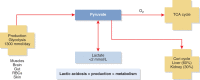
Acid-base disorders are common in the intensive care unit. By utilizing a systematic approach to their diagnosis, it is easy to identify both simple and mixed disturbances. These disorders are divided into four major categories: metabolic acidosis, metabolic alkalosis, respiratory acidosis, and respiratory alkalosis. Metabolic acidosis is subdivided into anion gap and non-gap acidosis. Distinguishing between these is helpful in establishing the cause of the acidosis. Anion gap acidosis, caused by the accumulation of organic anions from sepsis, diabetes, alcohol use, and numerous drugs and toxins, is usually present on admission to the intensive care unit. Lactic acidosis from decreased delivery or utilization of oxygen is associated with increased mortality. This is likely secondary to the disease process, as opposed to the degree of acidemia. Treatment of an anion gap acidosis is aimed at the underlying disease or removal of the toxin. The use of therapy to normalize the pH is controversial. Non-gap acidoses result from disorders of renal tubular H + transport, decreased renal ammonia secretion, gastrointestinal and kidney losses of bicarbonate, dilution of serum bicarbonate from excessive intravenous fluid administration, or addition of hydrochloric acid. Metabolic alkalosis is the most common acid-base disorder found in patients who are critically ill, and most often occurs after admission to the intensive care unit. Its etiology is most often secondary to the aggressive therapeutic interventions used to treat shock, acidemia, volume overload, severe coagulopathy, respiratory failure, and AKI. Treatment consists of volume resuscitation and repletion of potassium deficits. Aggressive lowering of the pH is usually not necessary. Respiratory disorders are caused by either decreased or increased minute ventilation. The use of permissive hypercapnia to prevent barotrauma has become the standard of care. The use of bicarbonate to correct the acidemia is not recommended. In patients at the extreme, the use of extracorporeal therapies to remove CO 2 can be considered.
Copyright © 2022 by the American Society of Nephrology.
Conflict of interest statement
A. Achanti reports having consultancy agreements with Chinook Therapeutics and Ad-board for atrasentan and reports receiving research funding from Cure Glomerulonephropathy Kaneka Medical America LLC sub-I LDL apheresis for FSGS and the Omeros IgA trial. H.M. Szerlip reports having consultancy agreements with Amarin, AstraZeneca, Echonous, and Relypsa; having an ownership interest in Amgen, Biomarin, Gilead, Merck, and Pfizer; receiving research funding from Akebia, Aurinnia, Bayer, Boehringer Ingelheim, and Fibrogen; receiving honoraria from AstraZeneca, GlaxoSmithKlein, LaJolla Pharmaceuticals, and Triceda; serving on the Acute Care Committee of the National Board of Medical Examiners, Medical Knowledge Self-Assessment Program 19 committee of the American College of Physicians, and as an author for UpToDate; receiving honoraria as a speaker for AstraZeneca, GSK, LaJolla, and Tricida; and having other interests or relationships with American College of Physicians, American Society of Nephrology, and National Kidney Foundation.
Figures
References
-
- Al-Jaghbeer M, Kellum JA: Acid-base disturbances in intensive care patients: Etiology, pathophysiology and treatment. Nephrol Dial Transplant 30: 1104–1111, 2015 - PubMed
-
- Stewart PA: Modern quantitative acid-base chemistry. Can J Physiol Pharmacol 61: 1444–1461, 1983 - PubMed
-
- Kurtz I, Kraut J, Ornekian V, Nguyen MK: Acid-base analysis: A critique of the Stewart and bicarbonate-centered approaches. Am J Physiol Renal Physiol 294: F1009–F1031, 2008 - PubMed
-
- Chong WH, Saha BK, Medarov BI: Comparing central venous blood gas to arterial blood gas and determining its utility in critically ill patients: Narrative review. Anesth Analg 133: 374–378, 2021 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

