SARS-CoV-2 infection in Africa: a systematic review and meta-analysis of standardised seroprevalence studies, from January 2020 to December 2021
- PMID: 35998978
- PMCID: PMC9402450
- DOI: 10.1136/bmjgh-2022-008793
SARS-CoV-2 infection in Africa: a systematic review and meta-analysis of standardised seroprevalence studies, from January 2020 to December 2021
Abstract
Introduction: Estimating COVID-19 cumulative incidence in Africa remains problematic due to challenges in contact tracing, routine surveillance systems and laboratory testing capacities and strategies. We undertook a meta-analysis of population-based seroprevalence studies to estimate SARS-CoV-2 seroprevalence in Africa to inform evidence-based decision making on public health and social measures (PHSM) and vaccine strategy.
Methods: We searched for seroprevalence studies conducted in Africa published 1 January 2020-30 December 2021 in Medline, Embase, Web of Science and Europe PMC (preprints), grey literature, media releases and early results from WHO Unity studies. All studies were screened, extracted, assessed for risk of bias and evaluated for alignment with the WHO Unity seroprevalence protocol. We conducted descriptive analyses of seroprevalence and meta-analysed seroprevalence differences by demographic groups, place and time. We estimated the extent of undetected infections by comparing seroprevalence and cumulative incidence of confirmed cases reported to WHO.
Prospero: CRD42020183634.
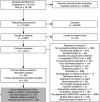
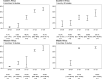
Results: We identified 56 full texts or early results, reporting 153 distinct seroprevalence studies in Africa. Of these, 97 (63%) were low/moderate risk of bias studies. SARS-CoV-2 seroprevalence rose from 3.0% (95% CI 1.0% to 9.2%) in April-June 2020 to 65.1% (95% CI 56.3% to 73.0%) in July-September 2021. The ratios of seroprevalence from infection to cumulative incidence of confirmed cases was large (overall: 100:1, ranging from 18:1 to 954:1) and steady over time. Seroprevalence was highly heterogeneous both within countries-urban versus rural (lower seroprevalence for rural geographic areas), children versus adults (children aged 0-9 years had the lowest seroprevalence)-and between countries and African subregions.
Conclusion: We report high seroprevalence in Africa suggesting greater population exposure to SARS-CoV-2 and potential protection against COVID-19 severe disease than indicated by surveillance data. As seroprevalence was heterogeneous, targeted PHSM and vaccination strategies need to be tailored to local epidemiological situations.
Keywords: COVID-19; epidemiology; public health; serology; systematic review.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: WHO had a role in study design, data collection, data analysis, data interpretation and writing of the report. No other funders had any such role. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. Authors were not precluded from accessing data in the study, and they accept responsibility to submit for publication. Potential other competing interests of named coauthors include: RKA reports personal fees from the Public Health Agency of Canada and the Bill and Melinda Gates Foundation Strategic Investment Fund, as well as equity in Alethea Medical (outside the submitted work). MPC reports personal fees from GEn1E Lifesciences (outside the submitted work), nplex biosciences (outside the submitted work) and Kanvas biosciences (outside the submitted work). JP reports grants from MedImmune (outside the submitted work) and Sanofi-Pasteur (outside the submitted work), grants and personal fees from Merck (outside the submitted work) and AbbVie (outside the submitted work) and personal fees from AstraZeneca (outside the submitted work). CCo reports funding from Sanofi Pasteur (outside of the submitted work). KE reports being cochairman of ANRS group on public health and social science in France (outside of the submitted work). JDS reports consulting fees from ASLM, GIZ health-focus and l’Oréal (all outside of the submitted work).
Figures


References
-
- Verhagen W, Bohl DK, Cilliers J. Long-Term socio-economic impacts of COVID-19 in African contexts | UNDP in Africa, 2021. Available: https://www.africa.undp.org/content/rba/en/home/library/reports/analysin... [Accessed 14 Jan 2022].
-
- World Health Organization . Data from: WHO coronavirus disease (COVID-19) dashboard, 2022. Available: https://covid19.who.int/
-
- World Health Organization . Strategic response to COVID-19 in the WHO African region, 2021. Available: https://www.afro.who.int/publications/strategic-response-covid-19-who-af... [Accessed 28 Apr 2022].
-
- Boakye-Agyemang C. Six in seven COVID-19 infections go undetected in Africa, 2021. Available: https://www.afro.who.int/news/six-seven-covid-19-infections-go-undetecte... [Accessed 14 Jan 2022].
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
